|
Biphasic Calcium Phosphate Contained within a Polyetheretherketone Cage with and without Plating for Anterior Cervical Discectomy and Fusion
Ralph Jasper Mobbs MD1,2, Anthony MT Chau MD2, Deniz Durmush MD2
1NeuroSpineClinic, Suite 7, Level 7 Prince of Wales Private Hospital; and 2Department of Spine Surgery, Prince of Wales Private Hospital, Sydney, Australia
- Objective: To evaluate the properties of a combination bone graft consisting of biphasic calcium phosphate ceramic, polyetheretherketone (PEEK) cage in one- and two-level surgery.
- Methods: Over a 12-month time period, a prospective single surgeon series of 75 patients were included in the study and 58 patients selected based on adequate data points. From these 58 patients, 32 were supplemented with anterior plate fixation and 26 patients without plating. Duration of clinical follow-up was a mean of 12.4 months (range, 6–26 months) in the Plated Group and 10.5 months (range, 6–21 months) in the Non-Plated Group.
- Results: A 100% fusion rate with nil graft related complications was achieved in the Plated group compared with 96.2% fusion and 11.5% subsidence rates reported in the Non-Plated group. Patients in both groups experienced statistically significant improvement in pain and functional outcomes compared to their pre-operative status; however, there was no significant difference in outcome between the Plated and Non-Plated Groups.
- Conclusions: Biphasic calcium phosphate ceramic contained within a PEEK cage is an effective implant for use in anterior cervical surgery with high fusion rates and good clinical outcome.
- Key words: Bone plates; Calcium phosphate; Cervical vertebrae; Spinal fusion
Introduction
The use of interbody fusion devices following anterior
decompression is a widely accepted procedure in patients
suffering degenerative or posttraumatic conditions of the cervical
spine. Degenerative disease of the cervical spine including
spondylosis, stenosis, herniated intervertebral discs and ossification
of the posterior longitudinal ligament can cause significant
radiculopathy and/or myelopathy1, resulting in functional
limitations, disability and loss of quality of life. The goals of
surgical intervention are decompression of neural elements
through removal of the pathological intervertebral disc structures
and restoration of spinal alignment and stability.
Anterior cervical discectomy and fusion (ACDF) is one
of the most commonly performed spinal procedures and has
good to excellent clinical results in the majority of cases in
treating cervical disc disease and associated radiculopathy and
myelopathy2,3. The advantages of an anterior approach are
minimal soft tissue injury, direct visualization of the spinal
cord and nerve roots to be decompressed, complete removal of
degenerative or traumatized intervertebral disc and access to
two endplates with a considerable surface area to facilitate
fusion4,5. According to one meta-analysis, fusion rates in
ACDF range from 92.1% to 94.6% in one- and two-level disc
disease1. Although complications are rare the most commonly occurring problems are isolated dysphagia,wound haematoma
and recurrent laryngeal nerve palsy2.
Address for correspondence Ralph Jasper Mobbs MD, Department of Spine Surgery, Prince of Wales Private Hospital, Barker St, Sydney, Australia 2031 Tel: +61 2 9650 4766; Fax: +61 2 9650 4943; Email: ralphmobbs@hotmail.com
Disclosure: The authors declare no conflict of interest. No benefits in any form have been, or will be, received from a commercial party related directly or indirectly to the subject of this manuscript.
Received 5 March 2012; accepted 10 June 2012
While autograft remains the gold standard in ACDF6,7,
the graft harvesting process can result in a range of complications
and short- and long-term morbidity, namely donor site
pain, haematoma, lateral cutaneous nerve palsy and infection8,9.
Allograft, which gained popularity in the efforts to
circumvent the need for autograft, has its own associated complications
including the risk of disease transmission, infection
and histocompatibility differences10. Graft collapse and
pseudoarthrosis were also seen in bone graft only fusion11.
Thus the impetus behind the creation of intervertebral cages
with bone graft substitute technologies has been to minimize
or eliminate autograft and allograft use with the aim of
improving clinical outcomes3,6.
In this study, we evaluate the properties and effectiveness
of a biphasic calcium phosphate ceramic contained within a
polyetheretherketone (PEEK) cage for one- and two-level cervical
arthrodesis.Another purpose of this study was to evaluate
the fusion rates and outcomes in patients with or without
internal plate fixation. Thus our aim in this preliminary report
is to ascertain the usefulness of these materials in anterior
cervical fusion and to determine whether there are any significant
differences in radiological and clinical outcomes between
the Plated and Non-plated Groups.
Materials and Methods
Patient Data
Over a 12-month time period, 75 patients were operated and
data prospectively collected. Seventeen patients were excluded
from the study due to inadequate follow-up. The operative
surgeon (RJM) has a large catchment area from regional Australia
and given the litany of distance, there was a large dropout
from the prospective cohort due to difficulty with follow-up.
All 17 patients were contacted via phone; however, they could
not return for a face-face consultation and therefore were
excluded. From 58 remaining patients, 32 patients were identified
as having undergone ACDF with plate fixation, 25 of
which were for one-level and seven for two-level disease. There
were 26 patients who had ACDF without plating, which
included 16 one-level and 10 two-level operations. There were
37 males and 21 females, with a mean age of 50.3 years (range,
21–81). There were 15 smokers, six people with diabetes and 10
workers compensation cases.Within the non-traumatic injury
patients, the mean preoperative symptom length was 11.9
months (range, 0.75–60 months). Both groups had similar
mean demographics for age (50.0 vs 50.6 years), symptom
length (12.5 vs 12.2 months), operative levels (C5/6 and C6/7
the most common operative levels) and time to follow-up.
Inclusion criteria were traumatic injury or degenerative
disc disease causing radiculopathy, myelopathy or radiculomyelopathy
and unresponsive to conservative treatment. One
patient who underwent one-level ACDF without plating suffered
from Klippel-Feil Syndrome affecting adjacent levels.
These data are summarized in Table 1.
Surgical Procedure
All patients were operated on by the same surgeon (RJM) and
interbody grafting with a biphasic calcium phosphate implant
contained within a PEEK cage. A modified Smith-Robinson
technique was used under general anaesthesia for all operations.
After a right antero-lateral incision, Caspar retracting
pins were positioned in the adjacent vertebral bodies for
adequate distraction. Under the direct observation of an operating
microscope, the removal of pathological disc was performed
using rongeurs and curettes. Osteophytes were
removed and the posterior longitudinal ligament divided. In
all cases, complete decompression and visualization of the
dura and nerve roots was achieved. Decortication of the vertebral
endplates was performed to optimize the bone-graft
interface.
A trial cage was inserted to confirmthe height of the disc
space. Biphasic calcium phosphate (KG Bone, Kasios Biomaterials,
Launaguet, France) was packed into the centre of the
PEEK cage. The interbody implant was inserted using forceps
and tapped into place (Fig. 1).
With the implant in place, anterior plate fixation was
inserted for the Plated Group. Antero-posterior and lateral
plain radiographs were obtained intraoperatively to check
correct positioning before wound closure. All Non-Plated were
advised to wear a cervical orthosis postoperatively for a period
of 6 weeks.
Interbody Graft
KG Bone (Kasios Biomaterials) is composed of biphasic
calcium phosphate (BCP): an amalgamation of two ceramics
already in use in the cervical spine–hydroxyapatite (HA) and
beta-tricalcium phosphate (b-TCP), combined respectively in
a 60/40 ratio to provide a biologically and biomechanically
stable graft with osteoconductive properties. KG Bone has a
fully interconnected architecture, with a mean porosity of 60%
and a 600 micron pore size, facilitating osteointegration. It is
supplied sterile by the manufacturer. KG Bone is specifically
designed to fit precisely into a corresponding cervical cage
made of PEEK (“Kage” cervical cage, Kasios Biomaterials) and
together they are implanted into the empty disc space. The
PEEK cage (Fig. 2) has an anatomical shaped design with
retention grooves that help anchor the graft once implanted
and discourage graft migration.
Outcome Measures
A prospective review of patient files and imaging was performed
to determine clinical and radiographic outcome following
anterior cervical spine surgery. Surgical and graft
complications, need for additional surgery/re-operation and
fusion rates were noted.
Radiographic fusion was assessed at every follow-up by
an independent radiologist. Plain radiographs were the first
choice of modality for radiographic assessment. Ethics board
approval was for X-ray studies, including flexion/extension
radiographs, for the assessment of fusion. Approval for CT
scan was given only if there was the suggestion or potential for
non-union. Radiographs were routinely taken intraoperatively
then at one day, 6 weeks, 3 months, 6 months and one year
postoperatively. Fusion was considered successful if bridging
bone incorporating the graft and adjoining endplates was
apparent (Figs 3,4), with additional loss of radiolucency, restoration
of interbody space and no hardware failure. Lack of
movement on flexion/extension X-rays were also used to
confirm status. We defined subsidence as a decrease in disc
space height of at least 3 mm, and movement as change in
anterior or posterior displacement of the graft by at least
3 mm12. If required, computed tomography (CT) was performed
to verify the fusion status of an operated level.
| TABLE 1 Demographic data of patients included in this study |
| Variables |
Plated group |
Non-plated group |
| Total number |
32 |
26 |
| Age (years) |
50.0 (22–81) |
50.6 (21–71) |
| Sex (M:F) |
25:7 |
12:14 |
| Tobacco smokers |
10 |
5 |
| Diabetics |
3 |
3 |
| Workers compensation |
2 |
8 |
| Previous cervical surgery |
1 |
6 |
| Neurological deficit |
|
|
| Radiculopathy |
27 |
23 |
| Myelopathy |
14 |
6 |
| Pathology |
|
|
| Degenerative disease |
24 |
25 |
| Trauma |
7 |
1 |
| Redo ACDF |
1 |
0 |
| Preoperative pain (VAS) |
7.9 ± 1.5 (n = 31) |
7.78 ± 1.1 (n = 26) |
| Preoperative ODI (%) |
52.0 ± 17.0 (n = 29) |
52.5 ± 15.5 (n = 25) |
| Symptom length (months) in non-traumatic patients |
12.5 (0.75–48) |
12.2 (0.5–60) |
| Bone mineral aspirate (BMA) |
18 |
20 |
| Number of levels |
|
|
| One level surgery |
7 |
10 |
| Operated levels |
|
|
| C3/4 |
4 |
3 |
| C4/5 |
2 |
6 |
| C5/6 |
17 |
13 |
| C6/7 |
16 |
12 |
| C7/T1 |
0 |
2 |
| ODI, Oswestry Disability Index; VAS, visual analog score |
Clinical outcome was assessed using a variety of parameters.
Patients were asked to quantify their overall pain on a
Visual Analog Scale (VAS) for pain ranging from 0 (no pain/
discomfort) to 10 (worst pain/discomfort imaginable) preand
postoperatively. Functional outcome was measured using
the Oswestry Disability Index (ODI). Patients were also
assessed according to Odom’s criteria13 (Table 2) for their
overall clinical outcome. Patient satisfaction with their procedure
was elicited using the Patient Satisfaction Index (PSI) as
described by Palit et al.14 (Table 3) at final follow up. Length of stay and time before return to work were recorded where
applicable.
Statistical Analysis
Descriptive data are represented as means ± standard deviation
(range, minimum–maximum). All datasets were tested for
normality with the D’Agostino and Pearson omnibus normality
test. Nonparametric data was analyzed using the Mann–
Whitney U-test and parametric unrelated data with the
unpaired t-test for comparison of the results between the
Plated and Non-Plated Groups. A paired t-test was used for
comparison between pre- and postoperative continuous variables
within patient groups. Statistical significance was set at
level of P < 0.05. All analyses and graphs were generated using
a commercial software package (GraphPad Prism version 5.01,
GraphPad Software, Inc., La Jolla, CA, USA).
Results
From 75 patients in the original dataset, 58 patients were
available for follow-up observation with adequate data
points. Duration of clinical follow-up was a mean of 12.4
months (range, 6–26 months) in the Plated Group and 10.5 months (range, 6–21 months) in the Non-Plated Group. In
both groups there were clear trends in clinical improvement
in terms of pain and functional outcomes at final clinical
assessment.
Fig. 1 Surgical technique including four steps:
discectomy and distraction of the interbody space (A),
microsurgical decompression (B), trial spacer for graft
choice(c), and insertion of interbody
polyetheretherketone (PEEK) cage (D).
Fig. 2 Photograph showing a polyetheretherketone (PEEK) cervical
cage (Kage) containing 60:40 hydroxyapatite : tricalcium phosphate
(HA:TCP) biphasic calcium phosphate (KG Bone).
Fig. 3 Fine-cut CT scan (A) demonstrating
fusion of a two-level graft with plating at 3
months post-operatively with pain-free range
of motion, and the day-1 postoperatively
corresponding neutral sagittal and
antero-posterior X-rays (B).
Fig. 4 X-ray of two-level polyetheretherketone (PEEK) cage fusion
without plating 6 months postoperatively demonstrating solid union
posterior and through the PEEK cage implants.
Radiological Outcomes
In the Plated Group, 100% fusion rate was achieved by 6
months postoperatively and remained unchanged throughout
patient follow-up. Grafts demonstrated no movement on flexion/extension X-rays (Fig. 5). There were no incidences of
radiological complications such as graft subsidence,movement
or fracture. One X-ray demonstrated a 2 mm loss of disc space
height; however, this did not qualify as graft subsidence and
there were no associated symptoms developing over the course
of 26 months of follow up.
| TABLE 2 The Odom clinical outcome scoring system |
| Excellent |
No complaint referable to cervical disease. Able to
perform daily occupation without impairment |
| Good |
Intermittent discomfort referable to cervical disease.
No significant interference with work. |
| Fair |
Subjective improvement in symptoms. Physical
activity significantly impaired. |
| Poor |
Worsening or no improvement |
Inferior radiological results were achieved in the Non-
Plated Group. Of the 26 patients, four patients (15.4%) experienced
delayed fusion (set at 3 months postoperatively). One
patient had a non-union (3.8%) of one level from a two-level
operation, which required re-operation. There were three cases
of graft subsidence (11.5%) and one graft migration (3.8%),
which required reoperation; however, fusion was achieved
within 3 months. In the plating group, subsidence experienced
was less than 3 mm in all patients. Additionally, one patient
showed evidence of new adjacent segment degeneration on
magnetic resonance imaging 9 months after a one-level ACDF
without plating.
Comparison of VAS
In the two groups, the average postoperative neck or arm pain
as measured by VAS showed significant relief (P < 0.0001)
when compared with the preoperative scores (Fig. 6), but no
significant difference of improvement in VAS scores was
observed between the two groups (P = 0.1836). Overall pain in
the Plated group improved on average from 7.9 preoperatively to 1.5 postoperatively, with a mean improvement of 6.5 ± 2.1
(range, 1–9), while in the Non-Plated group pain improved
from 7.8 to 2.2 on average with a mean improvement of 5.6 ±
2.8 (range, 0–10).
| TABLE 3 Patient satisfaction index (PSI) scoring system |
| 1 |
Surgery met my expectations |
| 2 |
I did not improve as much as I had hoped but I would
undergo the same operation for the same results |
| 3 |
Surgery helped but I would not undergo the same
operation for the same outcome |
| 4 |
I am the same or worse as compared to before surgerys |
Comparison of ODI
Three patients in the Plated Group and one patient in the
Non-Plated Group returned incomplete ODI questionnaires
either pre- or postoperatively, so they were excluded from this
subset analysis, although their Odom’s Criteria were good or
excellent according to their last consultation. The average ODI
score showed significant improvement with surgery (P <
0.0001) (Fig. 7); however, there was no significant difference in
improvement between the Plated and Non-Plated Groups (P =
0.9170).Mean improvement in ODI score for the Plated Group
was 34.3 ± 19.7 (range, 2–78) while in the Non-Plated Group
mean improvement was 34.9 ± 20.6 (range, 4–72).
Odom’s Criteria and PSI
No significant difference was found between the Plated and
Non-Plated groups when comparing their Odom’s criteria and
PSI. Ninety-seven per cent of Plated patients achieved either
excellent (n = 16) or good (n = 15) outcomes according to
Odom’s criteria, with one attaining a fair result. Comparatively,
of the Non-Plated Group, 77% achieved an excellent (n
= 14) or good (n = 5) outcome, with 23% only attaining a fair (n = 6) result and one patient having a poor result.Within the
Plated Group (n = 29), there were 21 patients with a PSI of 1,
six patients with a PSI of 2 and one patient with a PSI of 3.
Within the Non-Plated Group (n = 26), there were 14 patients
with a PSI of 1, seven patients with a PSI of 2, four patients
with a PSI of 3 and one patient with a PSI of 4. Hence, 93% of
Plated patients were satisfied with their surgical outcome (PSI
of 1 or 2) compared with 54% of Non-Plated patients. No
significant difference was found in length of hospital stay or
time to return to work.
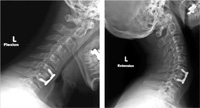 |
Fig. 5 F/E X-rays demonstrating a
mechanically stable one-level graft with
plate fixation.
Fig. 6 Preoperative and postoperative pain
rating (Visual Analog Scale [VAS]) in the
Plated (left) and Non-Plated (right) Groups.
Complications
Within the Plated group, there were two cases of dysphagia
(6.3%). One case of dysphagia lasted 3 months with only the
sensation of an “annoying lump in the throat” persisting at one
year postoperatively. One patient who had undergone a twolevel
Plated ACDF experienced ongoing dysphagia, with symptoms
only resolving following surgical removal of the plate.
One patient reported new neck pain after an initial good recovery
following an episode of severe vomiting involving rapid
flexion and extension. However, this complication selfresolved
after 4 weeks.
Fig. 7 Preoperative and postoperative
Oswestry Disability Index (ODI) scores in the
Plated (left) and Non-Plated (right) Groups.
Of the three cases of subsidence within the Non-Plated
Group, no re-operations were performed. One symptomatic
subsidence patient who refused re-operation improvedwithout
surgery. Another subsidence patient had only a 1 point improvement
in theirVAS score; however, their PSI was 2, indicating
that they “would undergo the same operation for the same
results”.This patient also experienced recurrent laryngeal nerve
palsy for 5months, likely sustained during the removal of a plate
from a previous adjacent level ACDF during this surgery. The
third subsidence patient presented for an ACDF re-operation
from 1 year prior. This patient experienced improvement in
radicular symptoms and pain; however, the patient developed
ongoing 5 out of 10 pain after 3months postoperatively, which
is being managedwith Gabapentin.Of note, all three of the graft
subsidence cases had undergone previous neck surgeries.
Graft migration was experienced in a non-plated ACDF
patient with complex cervical problems, including Klippel-Feil
Syndrome affecting adjacent levels, previous fusion surgery
and scoliosis, complicating their management. In this case
fusion was achieved within 3 months of re-operation and
although there was no accurately detectable improvement in
function (less than 10% improvement in ODI score15), there was improvement in pain and the patient rated their PSI as a 2.
One patient developed new symptoms, within 3 months postoperatively,
of parasthesias down the left arm and leg, exacerbated
by neck flexion. Nerve conduction studies indicated
irritation of the left posterior column, suggesting it was
sequelae of surgery. There were no instances of infection,
wound haematoma or chronic inflammation in either group.
The data are summarized in Table 4.
| TABLE 4 Summary of complications in plated and non-plated
groups |
| Complications |
Plated group |
Non-Plated group |
| Non-union |
0 (0%) |
1 (3.8%) |
| Graft subsidence |
0 (0%) |
3 (11.5%) |
| Graft migration |
0 (0%) |
1 (3.8%) |
| Dysphagia |
2 (6.3%) |
0 (0%) |
| Recurrent laryngeal nerve palsy |
0 (0%) |
1 (3.8%) |
| Adjacent disc disease (asymptomatic) |
0 (0%) |
1 (3.8%) |
Discussion
Autograft is still widely considered as the gold standard in
ACDF7. A Cochrane systematic review concluded that
fusion techniques using autograft yielded higher fusion rates
than allograft and synthetic bone substitute techniques;
however, other outcomes were not able to be assessed due to
the lack of standardized outcome measures within the literature4.
Hence, donor site morbidity associated with autograft
has fuelled the growing interest in alternative materials16,
namely ceramics, as fusion substrates for anterior cervical
arthrodesis. Ceramics provide a safe option with demonstrated
biocompatibility, osteoconductive potential, abundant and
affordable supply, and a means of avoiding morbidity at the
iliac crest. In this study, the combination of PEEK cage with
BCP, proved to be an effective and safe graft combination,
resulting in statistically significant improvements in pain and
function, both with and without plate fixation.
Graft Properties
PEEK is a semicrystalline polyaromatic linear polymer and
thermoplastic material of high molecular weight, which is biologically
inert, radiolucent and non-resorbable17. Moore and
Rhoad18 reported that PEEK elicits minimal cytotoxic and
inflammatory response from the host in a rat air pouch model.
Its other biomechanical properties include resistance to chemical
and radiation damage, compatibility with many reinforcing
agents (e.g. titanium, carbon fiber) and greater strength (per
mass basis) than manymetals19.Hence the PEEK cage provides
a hard frame able to resist spinal loading and has an elastic
modulus similar to that of bone, minimizing graft subsidence
and shrinkage20. It is also able to maintain spinal alignment
despite remodeling of bone graft within the cage cavity.
Radiological assessment of 42 patients who undertook
ACDF with a PEEK/TCP stand-alone cage achieved 94.5% fusion and 8.1% subsidence rate21. Cho et al. conducted a prospective
study of 40 patients who underwent one-level ACDF
with a PEEK/TCP cage versus iliac crest autograft. The PEEK/
TCP group achieved 100% solid fusion, increased cervical
lordosis and increased height and cross-sectional area of
foramina. Additionally, a minimal complication rate (2.5%
experienced pharyngitis) was noted in the PEEK group compared
with 17.5% complication rate in the autograft group
(including graft collapse, dislodgement and donor site morbidity)
20. Similar outcomes were achieved in a study comparing
PEEK cage filled with BCP to autograft, with the authors
deeming this graft combination a suitable alternative to
autograft, with shorter hospital stay, decreased operative time,
less blood loss and no donor site complications22,23.
The role of a PEEK/TCP combination without plating
was also compared to autograft with and without plate fixation
in multilevel ACDF24. By 12 months the PEEK/TCP cage
option and autograft with plate fixation demonstrated 100%
and 98% fusion rates, respectively, whereas autograft alone
achieved 87% fusion. Complication rates of autograft alone
were also much higher at 50% (owing to graft collapse,
pseudoarthrosis, dislodged graft) compared with 0% in the
PEEK/TCP group and 4% in autograft with plating. Overall,
the authors indicated preference for the PEEK/TCP cage in
treating multilevel cervical degenerative disease due to its significantly
lower complication rate.
Anterior Cervical Plating
Fixation plates reduce micromotion at the graft-host interface,
and resist graft settling and kyphotic deformity, but add to
costs, risks and operative time25.While anterior cervical plating
is commonly used to stabilize fusions and preserve disc space
height, there have also been reports of associated morbidity,
namely instrumentation failure26 (of which there are none in
this series) and dysphagia. The largest prospective study of
dysphagia following ACDF reported an overall incidence of
30% at 3 months postoperatively. Risk of dysphagia increased
with increasing number of operated levels and operative time;
however, no significant difference was found between Plated
and Non-Plated Groups27.
We believe there may be a role for plating in cagesupported
fusions as a precautionary measure against graft
subsidence. Gercek et al. reported on the use of a standalone
titanium cage to result in graft migration or subsidence in five
out of eight patients, with one patient experiencing symptom
recurrence and requiring reoperation12. Other authors have
noted relatively high rates of subsidence without plate use,
although with little correlation to clinical outcome6,28,29. A
meta-analysis of 21 studies by Fraser and Hartl revealed that
anterior plate systems significantly improve fusion rates in
one- and two-level disease, with a P < 0.00011.
As was demonstrated by our study, plate fixation in
one- and two- level disease may achieve earlier fusion and
decrease subsidence rates; however, this made no significant
difference to clinical outcomes. Our results were confirmed by
a prospective, randomized controlled trial with 2 years follow-up in which a PEEK cage filled with b-TCP was
assessed with and without plating. This study demonstrated a
significantly higher fusion rate at 3 months in the Plated
ACDF patients; however, all patients fused by 6 months. There
was also a significantly higher rate of graft migration in the
Non-Plated Group (P < 0.05), with 21.2% of Non-Plated
patients affected, compared to 0% of Plated patients. However
clinical outcomes were not significantly better than nonplating,
indicating that in one- and two-level ACDF, plate
fixation may not be necessary6.
Although the benefits and costs associated with internal
fixation in single-level fusions is contentious30, its use in these
procedures has nevertheless been found to be safe incurring no
increased complications31, or increased tendencies for adjacent
segment disease32. Plates also circumvent the need for cumbersome
external immobilization collars postoperatively and may
hasten patient recovery.
Limitations
A chief limitation of this study is the relatively small numbers
involved. Also, a number of patients have also had previous
surgeries performed in the neck region, which may have contributed
to their pathology. A review conducted by Hilibrand
andRobbins concluded that the prevalence of adjacent segment
disease is 13.6% at 5 years follow up, with the annual incidence
of adjacent segment disease requiring additional surgery being
between 1.5% and 4%33. Additionally, patients were not randomized
to treatment groups hence selection biases cannot be excluded. The uneven distribution of patients among the
groups also limits the statistical power of our conclusions.
Assessment of interbody fusion remains a challenge. As
there are no universally accepted criteria for determining
radiological fusion, it is often difficult to arrive at a true assessment
of fusion based on plain radiography alone particularly
when synthetic cages are used. Fine-cut CT scans with reconstructions
have been shown to be more reliable and sensitive
for the detection of pseudarthrosis than plain radiography34,35;
however, subjecting patients to CT scanning at regular intervals
purely for an assessment of fusion was deemed to be
unnecessary, costly and potentially harmful to patients. We
have only used CT scanning where there has been a query
regarding fusion status or pseudoarthrosis in the context of
unexpected/poor clinical outcomes and recurrence of symptoms
at follow up. X-ray with flexion/extension was performed
for the majority of patients (76%). The authors agree that this
may result in a higher apparent fusion rate, as compared with
a CT scan of every patient.
In this study, we have found that using a PEEK cage
containing BCP in one- and two-level anterior cervical discectomy
and fusion proved to be an effective treatment for cervical
spondylotic radiculopathy and/or myelopathy and is a
means of avoiding morbidity at the iliac crest. While anterior
plate fixation may promote early fusion rates and prevent cage
subsidence, no statistically significant difference was found in
clinical outcomes, (mean follow up was 11.5 months), when
compared with outcomes of non-plated ACDF patients.
References
- Fraser JF, Härtl R. Anterior approaches to fusion of the cervical spine: a
metaanalysis of fusion rates. J Neurosurg Spine, 2007, 6: 298–303.
- Fountas KN, Kapsalaki EZ, Nikolakakos LG, et al. Anterior cervical discectomy
and fusion associated complications. Spine, 2007, 32: 2310–2317.
- Moreland DB, Asch HL, Clabeaux DE, et al. Anterior cervical discectomy and
fusion with implantable titanium cage: initial impressions, patient outcomes
and comparison to fusion with allograft. Spine J, 2004, 4: 184–191.
- Jacobs WC, Anderson PG, Limbeek J, et al. Single or double-level anterior
interbody fusion techniques for cervical degenerative disc disease. Cochrane
Database Syst Rev, 2004, (4)CD004958.
- Thomas KA, Toth JM, Crawford NR, et al. Bioresorbable polylactide interbody
implants in an ovine anterior cervical discectomy and fusion model: three-year
results. Spine, 2008, 33: 734–742.
- Dai LY, Jiang LS. Anterior cervical fusion with interbody cage containing
beta-tricalcium phosphate augmented with plate fixation: a prospective
randomized study with 2-year follow-up. Eur Spine J, 2008, 17: 698–705.
- Chau AM, Mobbs RJ. Bone graft substitutes in anterior cervical discectomy
and fusion. Eur Spine J, 2009, 18: 449–464.
- Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior
iliac crest bone harvest for single-level anterior cervical discectomy and fusion.
Spine, 2003, 28: 134–139.
- Schnee CL, Freese A, Weil RJ, et al. Analysis of harvest morbidity and
radiographic outcome using autograft for anterior cervical fusion. Spine, 1997,
22: 2222–2227.
- Samartzis D, Shen FH, Goldberg EJ, et al. Is autograft the gold standard in
achieving radiographic fusion in one-level anterior cervical discectomy and
fusion with rigid anterior plate fixation? Spine, 2005, 30: 1756–1761.
- Hacker RJ, Cauthen JC, Gilbert TJ, et al. A prospective randomized
multicenter clinical evaluation of an anterior cervical fusion cage. Spine, 2000,
25: 2646–2654.
- Gercek E, Arlet V, Delisle J, et al. Subsidence of stand-alone cervical cages
in anterior interbody fusion: warning. Eur Spine J, 2003, 12: 513–516.
- Odom GL, Finney W, Woodhall B. Cervical disk lesions. J Am Med Assoc,
1958, 6: 23–28.
- Palit M, Schofferman J, Goldthwaite N, et al. Anterior discectomy and
fusion for the management of neck pain. Spine, 1999, 24: 2224–2228.
- Fairbank JC, Pynsent PB. The oswestry disability index. Spine, 2000, 25:
2940–2952.
- Vaccaro AR, Singh K, Haid R, et al. The use of bioabsorbable implants in
the spine. Spine J, 2003, 3: 227–237.
- Mastronardi L, Ducati A, Ferrante L. Anterior cervical fusion with
polyetheretherketone (PEEK) cages in the treatment of degenerative disc
disease. Preliminary observations in 36 consecutive cases with a minimum
12-month follow-up. Acta Neurochir (Wien), 2006, 148: 307–312.
- Moore R, Beredjiklian P, Rhoad R, et al. A comparison of the inflammatory
potential of particulates derived from two composite materials. J Biomed Mater
Res, 1997, 34: 137–147.
- Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal
implants. Biomaterials, 2007, 28: 4845–4869.
- Cho DY, Liau WR, Lee WY, et al. Preliminary experience using a
polyetheretherketone (PEEK) cage in the treatment of cervical disc disease.
Neurosurgery, 2002, 51: 1343–1349.
- Ha SK, Park JY, Kim SH, et al. Radiologic Assessment of Subsidence in
Stand-Alone Cervical Polyetheretherketone (PEEK) Cage. J Korean Neurosurg
Soc, 2008, 44: 370–374.
- Chou YC, Chen DC, Hsieh WA, et al. Efficacy of anterior cervical fusion:
comparison of titanium cages, polyetheretherketone (PEEK) cages and
autogenous bone grafts. J Clin Neurosci, 2008, 15: 1240–1245.
- Cho DY, Lee WY, Sheu PC, et al. Cage containing a biphasic calcium
phosphate ceramic (Triosite) for the treatment of cervical spondylosis. Surg
Neurol, 2005, 63: 497–504.
- Cho DY, Lee WY, Sheu PC. Treatment of multilevel cervical fusion with
cages. Surg Neurol, 2004, 62: 378–386.
- Rhee JM, Park JB, Yang JY, et al. Indications and techniques for anterior
cervical plating. Neurol India, 2005, 53: 433–439.
- Bose B. Anterior cervical fusion using Caspar plating: analysis of results
and review of the literature. Surg Neurol, 1998, 49: 25–31.
- Riley LH 3rd, Skolasky RL, Albert TJ, et al. Dysphagia after anterior cervical
decompression and fusion: prevalence and risk factors from a longitudinal
cohort study. Spine, 2005, 30: 2564–2569.
- Bartels RH, Donk RD, Feuth T. Subsidence of stand-alone cervical carbon
fiber cages. Neurosurgery, 2006, 58: 502–508.
- Mobbs RJ, Rao P, Chandran NK. Anterior cervical discectomy and fusion:
analysis of surgical outcome with and without plating. J Clin Neurosci, 2007,
14: 639–642.
- Samartzis D, Shen FH, Lyon C, et al. Does rigid instrumentation increase
the fusion rate in one-level anterior cervical discectomy and fusion? Spine J,
2004, 4: 636–643.
- Wang JC, McDonough PW, Endow K, et al. The effect of cervical plating on
single-level anterior cervical discectomy and fusion. J Spinal Disord, 1999, 12:
467–471.
- Rao RD, Wang M, McGrady LM, et al. Does anterior plating of the cervical
spine predispose to adjacent segment changes? Spine, 2005, 30:
2788–2793.
- Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent
segment disease: the consequences of spinal fusion? Spine J, 2004, 4 (6
Suppl.): 190S–194S.
- Carreon LY, Glassman SD, Djurasovic M. Reliability and agreement
between fine-cut CT scans and plain radiography in the evaluation of
posterolateral fusions. Spine J, 2007, 7: 39–43.
- Santos ER, Goss DG, Morcom RK, et al. Radiologic assessment of
interbody fusion using carbon fiber cages. Spine, 2003, 28: 997–1001.
 ACDF TCP Kage Orthopaedic Surgery ACDF TCP Kage Orthopaedic Surgery
You will need the Adobe Reader to view and print the above documents. 
|
