|
Phrenic nerve stimulation: The Australian experience
Peter Khong a,*, Amanda Lazzaro b, Ralph Mobbs a
- Department of Neurosurgery, Prince of Wales Hospital, Barker Street, Randwick, New South Wales 2031, Australia
- School of Medical Sciences, Faculty of Medicine, University of New South Wales Kensington Campus, Sydney, New South Wales, Australia
Abstract
Phrenic nerve stimulation is a technique whereby a nerve stimulator provides electrical stimulation of
the phrenic nerve to cause diaphragmatic contraction. The most common indications for this procedure
are central alveolar hypoventilation and high quadriplegia. This paper reviews the available data on the
19 patients treated with phrenic nerve stimulation in Australia to date. Of the 19 patients, 14 required
pacing due to quadriplegia, one had congenital central hypoventilation syndrome and one had brainstem
encephalitis. Information was unavailable for the remaining three patients. Currently, 11 of the pacers are
known to be actively implanted, with the total pacing duration ranging from 1 to 21 years (mean
13 years). Eight of the 19 patients had revision surgeries. Four of these were to replace the original I-
107 system (which had a 3–5-year life expectancy) with the current I-110 system, which is expected
to perform electrically for the patient's lifetime. Three patients had revisions due to mechanical failure.
The remaining patients' notes were incomplete. These data suggest that phrenic nerve stimulation can be
used instead of mechanical ventilators for long-term ongoing respiratory support.
© 2009 Elsevier Ltd. All rights reserved.
1. Introduction
Phrenic nerve stimulation is a technique whereby a nerve stimulator provides electrical stimulation of the phrenic nerve to cause diaphragmatic contraction. First described conceptually by Duchenne1 in 1872 as the ''best means of imitating natural respiration", the groundbreaking work came in the late 1960s by Glenn et al.2–4 –subsequently, in conjunction with Avery Biomedical Devices (Commack,NY, USA), the first phrenic nerve stimulators were brought into commercial distribution. Phrenic nerve stimulation has been practiced for several decades in Australia, with the first being performed in 1977; however, it remains relatively uncommon.
The two main indications for phrenic nerve stimulation are central alveolar hypoventilation and high quadriplegia. Of the former,
children suffering from congenital central hypoventilation syndrome (CCHS) form a unique group that often benefit drastically from this procedure. In the latter, patients typically with high cervical injuries (at or above C3) are the best candidates. The ultimate aim is to improve quality of life through temporary or permanent relief from the use of an artificial ventilation device.
Although phrenic nerve stimulation has now been described, and practiced, for several decades, it is still in its relative infancy, and there is still much work in innovation and advancement in this area. We describe the Australian experience of phrenic nerve stimulation in this series. To date, there have only been 19 patients who have undergone this procedure in Australia (Table 1).
2. Methods and device
The phrenic nerve stimulator consists of an electrode placed on the phrenic nerve and connected to a subcutaneous receiver via lead wires (Fig. 1). An external battery-operated transmitter sends radiofrequency energy to the receiver through an antenna, which is placed on the skin overlying the receiver. The receiver converts this energy into an electrical current that is directed to the phrenic nerve in order to stimulate the nerve, thereby causing contraction of the diaphragm.
The surgery can be performed via either a cervical or thoracic approach.
2.1. Cervical approach
A linear, horizontal skin incision is made and the sternocleidomastoid muscle is retracted medially. The phrenic nerve is identified
over the anterior scalenus muscle and isolated, and an electrode is attached (Fig. 2). The lead is tunnelled into a pocket in the anterior chest wall, and the receiver placed in a subcutaneous pocket.
2.2. Thoracic approach
This procedure is performed either via open thoracotomy at the 2nd or 3rd intercostal space, or thorascopically using trochars at
the 5th, 7th and 9th intercostal spaces along the posterior axillary line. The lungs are deflated one side at a time and the phrenic nerve
is mobilised over cardiac structures. The electrode is positioned
P. Khong et al. / Journal of Clinical Neuroscience 17 (2010) 205–208
Table 1
Details of the 19 patients with phrenic nerve stimulators implanted in Australia
| Patient |
Age (yrs),
sex |
Diagnosis |
Pacer status
(no. yrs) |
Location |
Reason for reoperation |
| 1 |
U, M |
Not on file |
? Active: Implanted |
Not on file |
N/A |
| 2 |
U, F |
Not on file |
Deceased: Unknown |
C unilateral (R) |
N/A |
| 3* |
35, F |
Quadriplegia |
Deceased: Implanted |
T bilateral |
Upgrade 5 yrs after initial surgery§ |
| 4 |
47, M |
Complete tetraplegia: C4–5 fracture
with ascending paralysis to C2–3 level |
Deceased: Implanted |
C unilateral (L) |
N/A |
| 5* |
30, F |
Quadriplegia |
Active: Implanted (21) |
C bilateral |
Upgrade 5 yrs after initial surgery§ |
| 6* |
28, M |
Quadriplegia |
Active: Implanted (21) |
T bilateral |
Upgrade 5 yrs after initial surgery§ |
| 7* |
38, F |
Quaplegia: C3–4 incomplete
quadriplegia |
Deceased: Unknown |
Not on file |
Upgrade 5 yrs after initial surgery§ |
| 8 |
63, F |
Quadriplegia: C1–2 fracture,
complete C2 quadriplegia |
Deceased: Unknown |
C bilateral |
N/A |
| 9* |
38, M |
Quadriplegia |
Active: Implanted (17) |
C bilateral |
Malfunction in pacer 4 yrs after initial surgery, upgraded§ |
| 10** |
19, F |
Quadriplegia: High cervical quadriplegia,
disrupted spinal cord at C1–2 level |
Active: Implanted (15) |
T bilateral |
Failure of both right and left receivers due to breast
development; receivers replaced in a more
inferior and superficial position |
| 11 |
66, F |
Not on file |
Deceased: Unknown |
T bilateral |
N/A |
| 12* |
15, M |
CCHS |
Deceased: Unknown |
T bilateral |
Not on file |
| 13 |
36, F |
Quadriplegia |
Active: Implanted (12) |
C bilateral |
N/A |
| 14* |
15, M |
Brainstem encephalitis |
Active: Implanted (12) |
C bilateral |
R lead replacement due to mechanical failure |
| 15 |
16, M |
Quadriplegia |
Active: Implanted (12) |
C bilateral |
N/A |
| 16 |
33, F |
Quadriplegia |
Active: Implanted (11) |
C bilateral |
N/A |
| 17 |
28, M |
Quadriplegia |
Active: Implanted (10) |
T bilateral |
N/A |
| 18 |
7, F |
Quadriplegia: Pneumococcal mastoiditis
complicated by cervicomedullary infarct |
Active: Implanted (3) |
C bilateral |
N/A |
| 19 |
24, M |
Quadriplegia |
Active: Implanted (1) |
C bilateral |
N/A |
C = cervical, CCHS = congenital central hypoventilation syndrome, F = female, L = left, M = male, N/A = not available, R = right, T = thoracic, U = age unknown, yrs = years.
* = reoperation.
** = reoperation twice.
§ = upgrade from I-107 to I-110 system.
below the nerve and sutured into place. The leads are brought
through the thoracic cavity and tunnelled into a subcutaneous
pocket inferior to the 12th rib, and the receiver is placed into this
pocket.
Generally, pacing is initiated four to six weeks post-operatively,
and gradually increased over several weeks.
2.3. Methods of analysis
We reviewed the available data on patients who have had phrenic
nerve stimulators implanted in Australia. These data were obtained
from Avery Biomedical Devices, who have been, and are
currently, the sole distributor of this device to Australia. The available
medical records were then obtained from the relevant hospitals
and any additional useful information was retrieved from
these, including infections, failure of device, lead migration and
longevity of stimulation.
3. Results
A total of 19 patients have had phrenic nerve simulators implanted
in Australia. The first of these was performed in 1977;
however, this patient has been lost to follow-up. Seven of the 19
patients have since died. Unfortunately the information regarding
cause of death was unavailable in all but one patient, who died
from pneumonia.
Eleven patients are still actively implanted, with total pacing
duration ranging from 1 year to 21 years. The average pacing duration
for actively pacing patients in whom records were available is
13 years. Several of the patients were either lost to follow-up or the
records were unobtainable.
In the 16 patients on whom information was available regarding
the original condition that required the use of phrenic nerve
stimulators, 14 were listed as having quadriplegia (most were traumatic,
although one was related to a cervicomedullary infarct following
pneumococcal mastoiditis), one patient suffered from
absent respiratory drive as a result of brainstem encephalitis, and
one patient had CCHS.
Eleven patients underwent cervical approaches, of which two
were unilateral and nine were bilateral. Six patients had thoracic
approaches, all of which were bilateral. There were two undocumented
approaches.
Eight patients had repeat operations for replacement/reimplantation
of hardware. The original I-107 receiver design was known
to have a 3-year to 5-year life expectancy, and four patients have
had re-implantations for this reason. The current I-110 receiver design
is expected to perform electrically for the patient's lifetime.
Of the reasons for the other replacement/reimplantations, one
patient's notes were not on file, and the other three were all related
to mechanical failure.
One patient experienced malfunction of the diaphragmatic
pacemaker 4 years after initial surgery, requiring ventilation at
home. Eventually, a I-110 pacer was used to replace the older I-
107 device. One patient required lead replacement on the right
side due to mechanical failure of implanted components – in the
interim, he required full ventilation during sleep for 1 month.
Another patient experienced failure of both left-sided and then
right-sided receivers due to breast development. The receivers
were replaced in a more inferior and superficial position (with ventilation
via tracheostomy used in the interim). In a recent followup
of this patient 15 years after the initial surgery, she was using
the pacing during the day and mechanical ventilation at night.
The left pacer was also noted to be less efficient – this was due
to difficulty in locating the antenna over the receiver due to weight
gain, and increasing the amplitude of the stimulating current of the
transmitter provided some improvement to this problem.
Of the patients on whom follow-up information was readily obtained,
several complications were noted in most. These were not unexpected, and typical of patients with quadriplegia. They included
recurrent respiratory tract infections, urinary tract infections,
pressure sores, kyphoscoliosis, neurogenic bladder and
muscle spasms.
P. Khong et al. / Journal of Clinical Neuroscience 17 (2010) 205–208
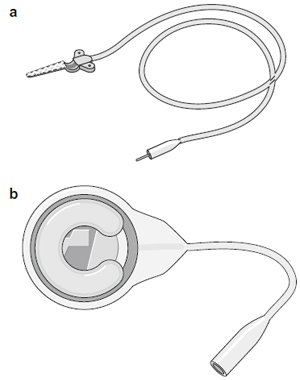
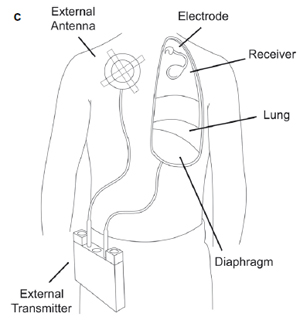
Fig. 1. The phrenic nerve stimulator system showing (a) a monopolar electrode, (b)
a I-110 receiver (Avery Biomedical Devices; Commack, NY, USA) and (c) location of
the system components.
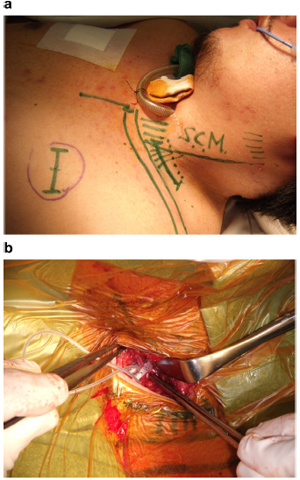
Fig. 2. (a) Surface marks on a patient indicating position of the incision (dotted line) in relation to the clavicle, sternocleidomastoid (S.C.M.), and the position of the subcutaneous receiver (broken circle). (b) Intraoperative photograph showing attachment of the electrode to the phrenic nerve via the cervical approach. This figure is available in colour at www.sciencedirect.com.
4. Discussion
Phrenic nerve stimulators can be implanted via two routes – a
cervical approach, or a thoracic approach. Initially, the thoracic approach
involved a thoracotomy, but more recently a less-invasive
thorascopic approach5 has been used successfully. Intramuscular
diaphragm stimulation is another technique described that aims
to cause less potential injury to the phrenic nerve through direct
stimulation of the diaphragm – however, electrode wires that exit
the skin carry a small but significant infection risk.6
Multiple complications may be associated with the implantation
of phrenic nerve stimulators. Complications involving the
hardware include mechanical failure, electrode failure or dislodgement,
and broken or disconnected wires – this can often result in
the replacement of the stimulator or reversion back to ventilatory
support. Lung complications including atelectasis, pneumonia and
pneumothorax are all possible with the thoracic approach.5 Infection
is also a potentially serious complication which may require
removal of the affected device. Damage to the phrenic nerve may
occur acutely during surgery and render a phrenic nerve stimulator
ineffective – there are also questions as to whether chronic, long term stimulation itself may damage the nerve over time or even
cause diaphragmatic failure, athough results thus far have been
positive and there is no evidence to support this.
Weese-Mayer et al.7 published a review of the international
experience with quadruple diaphragm pacer systems, which included
35 children and 29 adults. They noted that 2.9% of patients
experienced infection, and 3.8% experienced mechanical trauma.
Presumed electrode and receiver failure occurred in 3.1% and
5.9% of patients with tetraplegia and CCHS respectively. Overall,
the figures were overwhelmingly positive, with 94% of paediatric
patients pacing successfully, 60% of these complication free, and
86% of adult patients pacing successfully, 52% of these complication
free.
Garrido-Garcia et al.8 published a series in 1998 on 22 patients
treated with diaphragmatic stimulators: 18 patients achieved permanent
pacing, and the remaining four required pacing only during
sleep. One patient had phrenic nerve entrapment by scar
tissue and four experienced infections, all of whom required operative
reimplantation. Pacemaker complications included antenna
fractures and receiver failure. Five patients died during followup.
Although the mean duration of follow-up was only 3 to
4 months, one patient was followed up for 11 years and four for
10 years, indicating that it may be possible for diaphragmatic pacing
to achieve complete stable long-term ventilation.
Shaul et al.5 successfully implanted phrenic nerve stimulators
through a thoracoscopic approach. Nine patients, all children, were
described. Over a mean follow-up period of 30 months, eight patients
reached their long-term pacing goals. Four patients experienced
post-operative complications (pneumonia, atelectasis,
bradycardia and pneumothorax), with the recognition that aggressive
post-operative pulmonary hygiene was required.
Elefteriades et al.9 published long-term pacing results on 12 patients
with quadriplegia: six of 12 patients continued full-time
pacing with a mean of 14.8 years. Patients who stopped full-time
pacing did so due to social/financial reasons or medical comorbidities
rather than complications directly related to the phrenic nerve
stimulators themselves. They also pointed out that there was no
evidence to suggest long-term nerve injury could result from
chronic pacing, with no apparent clinical deterioration in pacing
parameters or respiratory measurements from continuous pacing
for over 10 to 15 years.
B. M. Soni, in his article "Use of phrenic nerve stimulator in high
ventilator dependent spinal cord injury" (P. Khong, pers. comm..
2009) reviewed 20 ventilator-dependent patients with high cervical
spinal cord injuries who had undergone phrenic nerve stimulator
implantation. One paediatric patient failed to produce adequate
tidal volumes with stimulation; one patient developed a cable fracture
requiring conversion of the system to an intrathoracic stimulator,
and 18 of the 20 patients reported significant benefit in
mobility, access and overall improvement in quality of life.
More recently, Hirschfeld et al.10 conducted a prospective study
comparing the outcomes of 64 spinal cord-injured patients who
were respiratory device-dependent. Half had functioning phrenic
nerves and diaphragm muscles and were treated with phrenic
nerve stimulators, and the other half with destroyed phrenic nerves were mechanically ventilated. They found that those treated
with phrenic nerve stimulators had a reduced frequency of
respiratory tract infections and improved quality of speech – these
results were statistically significant. Subjectively, they felt that
those with stimulators had improved quality of life.
In our case series, we found a total of 19 patients in whom phrenic
nerve stimulators have been implanted in Australia: 11 patients
had undergone cervical approaches and six had thoracic
approaches – this largely reflected surgeon preference, and to date
there are no conclusive data to show whether one approach is better
than another. Of interest, eight patients had to undergo reimplantations
– four were expected due to the 3-year to 5-year life
expectancy of the original I-107 receiver design, three were due
to mechanical failure (one patient's notes were not available).
5. Conclusion
To the time of writing, 19 patients have had phrenic nerve stimulators
implanted in Australia. Although the devices have been
available for several decades, their use is still regarded as specialised
and uncommon, especially in Australia. We acknowledge that
complications can arise attributable to mechanical failure, as well
as the expected complications inherent in patients with quadriplegia.
Of the patients known to be actively pacing, the average duration
of ongoing pacing is 13 years – this suggests that phrenic
nerve stimulators can be used in the long term instead of mechanical
ventilators for ongoing respiratory support. Follow-up studies
will be valuable in determining whether phrenic nerve stimulators
can be a permanent solution to the respiratory issues related to
central alveolar hypoventilation and high quadriplegia.
References
- Duchenne GB. De l'electrisation localisee et de son application a la pathologie et la
therapeutique par courants induits at par courants galavaniques interrompus et
conius. 3rd ed. Paris: J.B. Bailliere et Fils; 1872. pp 907–18.
- Judson JP, Glenn WWL. Radio-requency electrophrenic respiration. Long-term
application to a patient with primary hypoventilation. JAMA 1968;203:1033–7.
- Glenn WWL, Phelps ML, Elefteriades JA, et al. Twenty years of experience in
phrenic nerve stimulation to pace the diaphragm. Pacing Clin Electrophysiol
1986;9:780–4.
- Glenn W, Brouillett R, Dentz B, et al. Fundamental considerations in pacing of
the diaphragm for chronic ventilatory insufficiency; a multicentre study. Pacing
Clin Electrophysiol 1988;11:2121–7.
- Shaul DB, Danielson PD, McComb JG, et al. Thoracoscopic placement of phrenic
nerve electrodes for diaphragmatic pacing in children. J Pedriatr Surg
2002;37:974–8.
- DiMarco AF, Onders RP, Ignagni A, et al. Phrenic nerve pacing via intramuscular
diaphragm electodes in tetraplegic subjects. Chest 2005;127:671–8.
- Weese-Mayer DE, Silvestri JM, Kenny AS, et al. Diaphragm pacing with a
quadripolar phrenic nerve electrode; an international study. Pacing Clin
Electrophysiol 1996;19:1311–9.
- Garrido-Garcia H, Alvarcz JM, Escribano PM, et al. Treatment of chronic
ventilatory failure using a diaphragmatic pacemaker. Spinal Cord 1998;36:
310–4.
- Elefteriades JA, Quin JA, Hogan JF, et al. Long-term follow-up of pacing of the
conditioned diaphragm in quadriplegia. Pacing Clin Electrophysiol 2002;25:
897–906.
- Hirschfeld S, Exner G, Luukkaala T, et al. Mechanical ventilation or phrenic
nerve stimulation for treatment of spinal cord injury-induced respiratory
insufficiency. Spinal Cord 2008;46:738–42.
 Phrenic Nerve Stimulation Phrenic Nerve Stimulation
You will need the Adobe Reader to view and print the above documents. 
|
