|
Reconstruction versus no reconstruction of iliac crest defects following harvest for spinal fusion: a systematic review
A review
Anthony M. T. Chau, M.B.B.S. (Hons.),1 Lileane L. Xu,2 Rhys van der Rijt, M.B.B.S.,3
Johnny H. Y. Wong, M.B.B.S. (Hons.), M.Med.,1,4 Cristian Gra gnaniell o, M.D.,4
Ral ph E. Stanford, M.B.B.S., Ph.D., F.R.A.C.S.,2,5
and Ral ph J. Mobbs , M.B.B.S., M.S., F.R.A.C.S.2,6
1Department of Neurosurgery, Royal Prince Alfred Hospital; 2Faculty of Medicine, University of New South
Wales; 3Department of Neurosurgery, St. Vincent's Hospital; 4Department of Neurosurgery, Australian School
of Advanced Medicine, Macquarie University Hospital; 5Department of Orthopaedics, Prince of Wales
Hospital; and 6Department of Neurosurgery, Prince of Wales Hospital, Sydney, Australia
Object: Autologous bone from the iliac crest is commonly used for spinal fusion. However, its use is associated
with significant donor site morbidity, especially pain. Reconstructive procedures of the iatrogenic defect have been
investigated as a technique to alleviate these symptoms. The goal of this study was to assess the effects of reconstruction
versus no reconstruction following iliac crest harvest in adults undergoing spine surgery.
Methods: The authors searched the Cochrane Central Register of Controlled Trials (The Cochrane Library 2011,
Issue 4); MEDLINE (1948–Oct 2011); EMBASE (1947–Oct 2011); and the reference lists of articles. Randomized
controlled trials (RCTs) or nonrandomized controlled trials (NRCTs) were included in the study. Two independent
reviewers selected the studies, extracted data using a standardized collection form, and assessed for risk of bias.
Results: Three RCTs (96 patients) and 2 NRCTs (82 patients) were included. These had a moderate to high risk
of bias. The results suggest that iliac crest reconstruction may be useful in reducing postoperative pain, minimizing
functional disability, and improving cosmesis. No pattern of other clinical, radiological, or resource outcomes was
identified.
Conclusions: Although the available evidence is suboptimal, this systematic review supports the notion that iliac
crest reconstruction following harvest for spinal fusion may reduce postoperative pain, minimize functional disability,
and improve cosmesis.
(http://thejns.org/doi/abs/10.3171/2012.3.SPINE11979)
Key Words :: systematic review :: reconstructive surgery :: bone substitute
bone transplantation :: ilium :: postoperative complication :: spinal fusion
Abbreviations used in this paper: CHA = coralline hydroxyapatite;
ICM = inductive conductive matrix; NRCT = nonrandomized
controlled trial; RCT = randomized controlled trial; SF-36 = 36-Item
Short Form Health Survey; VAS = visual analog scale.
Autologous bone from the iliac crest has for decades
been harvested as a substrate for spinal
arthrodesis. Unfortunately, donor site morbidity
and the requirement for a second surgical site have been
major detriments for its unhindered use.6 Whereas most
donor site complaints usually resolve with time, a number
of patients unfortunately experience persisting symptoms
that become a significant source of postoperative morbidity.
Chronic pain at the iliac crest is arguably the most significant
complication, occurring in approximately 20% of
patients.20,26,28 The mechanism of the pain is unclear, but
has been speculated to be due to trauma to the periosteum
or muscle or to neuroma formation.32 Additionally, others have suggested that patients undergoing lumbar surgery
may have confusing, referred pain.9,18 A wide range of
other complaints, including quality of life and cosmetic
issues, have also been documented.20,26,28
Despite this, autograft from the iliac crest remains
the gold standard substrate because currently no substitute
supersedes its combined osteogenic, osteoinductive,
and osteoconductive potential in promoting bony fusion.6
For many surgeons, especially those in countries where
cost is an additional significant constraint, autograft remains
a reliable, attractive, and easily accessible option,
and so its utility remains widespread.4 Autograft from
other sites, notably local decompressed bone, has also become
widely used. In certain procedures, such as singlelevel
posterolateral lumbar fusion, local bone has been
shown to be equivalent to iliac crest harvest, although its
success decreases with increasing levels.27
While research into bone graft substitutes such as efforts have concurrently been made to support iliac crest autograft procedures by developing methods to alleviate donor site morbidity. Medical therapies that have been investigated include intraoperative31 and continuous
postoperative anesthetic administration,22,29 which have achieved mixed results.
Surgically, many techniques such as rounding of
bony edges,30 harvesting from the posterior instead of the
anterior iliac crest,2 and minimally invasive procedures,
among others, have been suggested.24 Finally, reconstruction
of the iatrogenic defect, first proposed by Hardy in
1977,14 has been investigated. Similar to the unknown
mechanism of donor site pain, the way in which reconstruction
may alleviate morbidity is also unclear.32
Currently, no systematic synthesis of the efficacy of this "backfill" procedure exists in the literature. Hence, the aim of this review is to assess the effects of reconstruction versus no reconstruction of iliac crest harvest defects in adult humans undergoing spine surgery.
Methods
Types of Studies
All prospective controlled human clinical studies
(level III-2 evidence or higher) were considered (see Table
1 for hierarchy of evidence). This included a search for
NRCTs as well as RCTs, in anticipation of a low number
of the latter.
Types of Participants
We considered male and female adult patients who
underwent
iliac crest harvest as a donor site for spinal surgery.
Types of Interventions
We compared the intervention of iliac crest reconstruction
versus no reconstructive procedure following
iliac crest harvesting. We considered all types of reconstructive
material, including autologous, allogeneic, and
synthetic materials.
Outcomes Assessed
The primary outcome assessed was the effect on
donor site pain. Secondary outcomes included quality
of life/functional disability, cosmetic appearance, radiological
analysis, resource use (such as hospital stay), and
any notable complications (such as skin necrosis, bursitis,
meralgia paresthetica, herniation, and gait disturbance).
Search Strategy
A literature search of the Cochrane Central Register
of Controlled Trials (The Cochrane Library 2011, Issue
4); MEDLINE (via Ovid) (1948–Oct 2011); and EMBASE
(Ovid) (1947–Oct 2011) was performed in Week 3, October
2011. The specific prospective search protocol for
each database is outlined in Table 2. No language restrictions
were used. In addition, the reference lists and citation
history of all full-text articles retrieved were checked
using Scopus. A search for ongoing or recently completed
trials was performed in Week 1, November 2011 in the US Clinical Trials (www.clinicaltrials.gov), UK Current
Controlled Trials (www.controlled-trials.com), and Australian
New Zealand Clinical Trials (www.anzctr.org.au)
registries by using similar keywords.
All titles were assessed, and where the abstract suggested
a potentially eligible study, the full text was retrieved.
Studies were critically evaluated for design and
risk of bias, and were classified according to level of evidence
(Table 1).23 Data were extracted onto a standardized
collection form by 2 independently working authors
(A.C. and L.X.) and entered into RevMan version 5.1.4.
TABLE 1: National Health and Medical Research Council hierarchy of evidence
| Level |
Study Design |
| I |
systematic review of level II studies |
| II |
RCT |
| III-1 |
pseudo-RCT |
| III-2 |
comparative study w/ concurrent controls |
| III-3 |
comparative study w/o concurrent controls |
| IV |
case series |
Results
Evidence Base
Included Studies: The literature search returned the following number of articles: CENTRAL (87), MEDLINE (512), and EMBASE (494) (Fig. 1). From these, 5 articles were found to be appropriate for the clinical question, of which 3 were RCTs5,25,33 and 2 were NRCTs
(Table 3).4,12 The indications for iliac crest harvesting in these studies were all related to spinal fusion. No studies from other disciplines such as oral maxillofacial surgery were located. The study by Yang et al.33 was translated into English and evaluated.
Excluded Studies: The search strategy yielded 1 controlled study, which was excluded because it was retrospective.32 A number of other studies were excluded due to their retrospective nature and/or lack of controls. 1,3,7,8,11,13–15,17,19,21 At the time of this writing, 1 prospective
NRCT had recently completed recruitment and was not yet available for analysis (see http://clinicaltrials.gov/ct2/show/NCT00837473).
Critical Appraisal of Included RCTs: Descriptions of
each included RCT are provided in Tables 4–6. The risk
of bias was assessed according to the guidelines set out in
the Cochrane Handbook (Table 7).16
Critical Appraisal of Included NRCTs: Descriptions
of the NRCTs are provided in Tables 8 and 9. The risk
of bias was assessed according to the validated checklist
developed by Downs and Black (Table 10).10
Statistical Analysis
Given the small number of patients from RCTs and
the heterogeneous interventions, a meta-analysis of data
was not performed.
TABLE 2: Literature search strategy, using MeSH (CENTRAL, MEDLINE) and Emtree (EMBASE) terms*
| No. |
CENTRAL |
MEDLINE |
EMBASE |
| 1 |
MeSH descriptor reconstructive surgical procedures
explode |
exp reconstructive surgical procedures |
exp plastic surgery |
| 2 |
MeSH descriptor bone transplantation explode all
trees |
exp bone transplantation |
exp bone transplantation |
| 3 |
MeSH descriptor bone substitutes explode all trees |
exp bone substitutes |
exp bone prosthesis |
| 4 |
MeSH descriptor prostheses and implants explode |
prostheses and implants.mp |
prostheses and implants.mp |
| 5 |
#1 or #2 or #3 or #4 |
or/1-4 |
or/1-4 |
| 6 |
MeSH descriptor ilium explode |
exp ilium |
exp iliac bone |
| 7 |
#5 AND #6 |
(iliac adj1 crest).mp. |
(iliac adj1 crest).mp. |
| 8 |
|
or/6-7 |
or/6-7 |
| 9 |
|
5 and 8 |
5 and 8 |
| 10 |
|
exp clinical trial |
clinical trial |
| 11 |
|
comparative study |
exp comparative study |
| 12 |
|
exp prospective studies |
exp prospective studies |
| 13 |
|
or/10-12 |
or/10-12 |
| 14 |
|
9 and 13 |
9 and 13 |
| 15 |
|
exp animals/ not humans.sh |
exp animals/ not humans.sh |
| 16 |
|
14 not 15 |
14 not 15 |
* adj1 = adjacent within 1 word; exp = explode search; mp = multiple posting (search term considered in the title, abstract, or
subject heading); sh = subject heading.
Surgical Technique
Anterior Harvest. Yang et al.33 harvested tricortical
bone from the anterior superior iliac spine, approximately
3.5–6 cm deep and 2.5–3 cm in thickness, by using an oscillating
saw and osteotome. The control group received bone wax for hemostasis. The reconstruction group received
selected autologous rib segments with the most appropriate
contour, followed by bone wax. The reasons for
harvest were anterior thoracic or lumbar fusion.
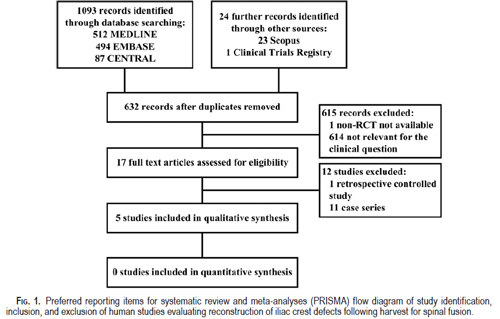
TABLE 3: Summary of included studies*
| Authors & Year |
Country |
Study Design |
Evidence Class |
Intervention |
Source |
| Yang et al., 2009 |
China |
RCT |
II |
autograft rib |
CENTRAL, MEDLINE, EMBASE |
| Bojescul et al., 2005 |
US |
RCT |
II |
CHA† |
CENTRAL, MEDLINE, EMBASE |
| Resnick, 2005 |
US |
RCT |
II |
β-TCP |
CENTRAL, MEDLINE, EMBASE |
| Bapat et al., 2008 |
India |
NRCT |
III-2 |
autograft rib |
MEDLINE, EMBASE |
| Epstein & Hollingsworth, 2003 |
US |
NRCT |
III-2 |
mesh & ICM |
CENTRAL, MEDLINE |
* β-TCP = beta–tricalcium phosphate.
† Pro Osteon 500 in this study.
Resnick25 harvested varying required amounts of tricortical
bone 2–4 cm lateral to the anterior superior iliac spine, 12–16 mm deep. Irrigation with antibiotics and saline
followed by cautery for hemostasis was applied. The
control group received Gelfoam hemostatic agent, whereas
the investigative group received packed morcellized
tricalcium phosphate. The reasons for harvest were 1- or
2-level anterior cervical discectomy and fusion, or 1-level
cervical corpectomy.
Bapat et al.4 harvested varying required amounts of
tricortical bone 3 cm posterior to the anterior superior
iliac spine. Sharp cortical edges were rounded off. Irrigation
and hemostasis were applied. In the investigative
group, 5-mm notches were prepared on either side of the
defect. A section of rib with the most appropriate contour
was then chosen, excised, and implanted into the iliac defect with impaction into the notches. The reasons for harvest
varied between groups, and are outlined in Table 8.
Epstein and Hollingsworth12 harvested an average
of 3-cm-long strut grafts from the left anterior iliac crest
by using an oscillating saw and curved osteotome (N.
E. Epstein, personal communication, 2012). The first 23
patients had bone wax applied to the cancellous surface.
The next 23 patients received iliac crest reconstruction in
which a MacroPore polymer sheet (MacroPore, Inc.) and
ICM (Sofamor Danek), a form of demineralized bone matrix
with minute amounts of bone morphogenetic protein
suspended in gel, were used. The ICM gel was warmed
and applied into the iliac crest defect, followed by application
of the contoured MacroPore sheet. Two resorbable
screws were placed on either side of the cortical shelves.
The reasons for harvest were for single-level anterior cervical
corpectomy and fusion.
TABLE 4: Characteristics of Yang study*
| Characteristic |
Data |
| methods |
RCT; duration 1 yr |
| participants |
- mean age 42 yrs (range not stated)
- eligibility criteria: ant thoracic or lumbar surgery, w/ ant
iliac crest harvest
- exclusion criteria: inappropriate ribs on preop x-ray,
severe neurological deficit, preexisting hip pain or
discomfort, mental illness
- total recruited: 54 pts (masking not stated); 39M:15F
|
| intervention |
autograft rib; 25 pts |
| comparison |
no iliac crest reconstruction, hemostatic bone wax; 29
pts |
| outcomes |
primary outcomes:
- donor site pain measured using VAS when lying still
on op side, & when active (2 wks, 3 mos postop);
pain measured using VAS when sleeping on op side
(1 yr)
- pt satisfaction w/ cosmesis (unsatisfied, moderately
satisfied, satisfied) (1 yr)
- pt comfort when wearing a belt w/ activity (uncomfortable,
moderately comfortable, comfortable) (1
yr)
- any clinical complications including fractures (w/in
1 yr)
(masking not stated)
|
| loss to FU |
none |
| funding/COI |
no declaration |
* Ant = anterior; COI = conflict of interest; FU = follow-up; pts = patients.
Posterior Harvest. Bojescul et al.5 used the "trapdoor" technique for their posterior iliac crest harvest. A 2 × 4 × 2–cm cortical window was created 8 cm from the
TABLE 5: Characteristics of Resnick study
| Characteristic |
Data |
| methods |
single-blinded RCT; duration 3 mos |
| participants |
- mean age 45 yrs (range not stated)
- smokers (29 of 30)
- eligibility criteria: 1- or 2-level ant cervical discectomy &
fusion, or 1-level cervical corpectomy, w/ ant iliac
crest harvest
- exclusion criteria: not stated
- total recruited: 30 pts (blinded); 19M:11F
|
| intervention |
morcellized β-TCP; 15 pts |
| comparison |
no iliac crest reconstruction, Gelfoam hemostatic agent;
15 pts |
| outcomes |
- primary outcomes: pain (immediately preop, & 24 hrs,
6 wks, & 3 mos postop) measured w/ patient-reported
McGill pain questionnaire (acute pain) & VAS (generalized
pain)
- secondary outcomes: plain x-ray appearance, cosmesis
as determined by surgeon (not blinded)
(masking not stated)
|
| loss to FU |
none |
| funding/COI |
supported by Orthovita, Inc. (Malvern, PA; manufacturer
of the intervention) |
Iliac crest reconstruction following harvest for spinal fusion
TABLE 6: Characteristics of Bojescul study
| Characteristic |
Data |
| methods |
double-blinded RCT; duration 1 yr |
| participants |
- mean age 35 yrs (range 23–67 yrs)
- eligibility criteria: spinal fusion, w/ posterior iliac crest
harvest
- exclusion criteria: pregnancy, peripheral vascular disease,
localized or systemic disease
- total recruited: 12 pts (blinded); 10M:2F
|
| intervention |
CHA; 5 pts |
| comparison |
no iliac crest reconstruction; 7 pts |
| outcomes |
- primary outcomes: donor site pain (predischarge, & at
6 wks, 3 mos, 6 mos, & 1 yr postop) measured w/
questionnaire (0, none; 1, mild; 2, medium; 3, moderate;
4, severe) and clinical palpation
- secondary outcomes: plain x-ray, CT, & SPECT appearance,
determined by 2 blinded observers
(masking not stated)
|
| loss to FU |
1 pt from each group |
| funding/COI |
none |
harvested. Patients in the investigative group received
sterile blocks and granulated CHA (Pro Osteon Implant
500). The reason for harvest was for spinal fusion (no further
details given).
Primary Outcome: Donor Site Pain
All studies examined donor site pain as a primary
outcome. Yang et al.33 reported decreased donor site pain
at 2 weeks and 3 months in patients with reconstruction
compared with those without reconstruction when active
(p < 0.05), but not when at rest (p > 0.05). At 1 year,
patients with reconstruction experienced less pain when
sleeping on the operative side (p < 0.05).
Resnick25 observed that the pain scores of patients
with reconstruction were significantly lower than those
in the control group in terms of both number and severity at the 6-week mark (p < 0.001). However, by 3 months,
as the pain scores of the group without reconstruction
diminished, a significant difference could no longer be
detected. The trend was only significant for the results of
the McGill pain questionnaire, but not the VAS.
TABLE 7: Assessment of potential bias in included RCTs*
| Criteria |
Yang et al. |
Resnick |
Bojescul et al. |
| random sequence generation (selection bias) |
+ |
? |
? |
| allocation concealment (selection bias) |
? |
? |
? |
| blinding of participants (performance bias) |
? |
+ |
+ |
| blinding of personnel (performance bias) |
? |
− |
+ |
| blinding of outcome assessment (detection bias) |
? |
+ |
+ |
| incomplete outcome data (attrition bias) |
+ |
+ |
− |
| selective reporting (reporting bias) |
+ |
+ |
− |
| other potential sources of bias |
4 cases of Pott disease
in rib group |
almost all smokers |
age difference, very small nos. |
| summary assessment of risk of bias |
moderate |
moderate |
high |
* Using criteria as set out by Higgins and Green in the Cochrane Handbook. + = low risk of bias; − = high risk of bias; ? = unclear,
method not stated.
Bojescul et al.5 reported the donor site pain results at 1 year only, although they had also assessed pain at earlier time points (1 patient in each group was lost to follow-up). Three of 4 patients who underwent reconstruction reported no pain, and 1 reported mild pain. Of
the 6 patients without reconstruction, 2 subjectively had no pain, 1 had mild pain, in 2 it was medium, and in 1 moderate. Objective pain analysis yielded similar results, and did not reveal any statistical significance (p = 0.199)
TABLE 8: Characteristics of Bapat study
| Characteristic |
Data |
| methods |
NRCT; duration 1 yr
allocation according to surgical approach |
| participants |
- mean age 39 yrs (range not stated)
- eligibility criteria: spinal fusion of ant column, w/ ant
iliac crest harvest
- exclusion criteria: iliac defects < 25 mm, incomplete
neurological recovery, persistent sensory abnormalities
- total recruited: 36 pts (masking not stated); 10M:26F
|
| intervention |
autogenous rib graft; 20 pts (thoracotomy [16] or thoracophrenicolumbotomy
[6]) |
| comparison |
no iliac crest reconstruction; 16 pts (ant cervical corpectomy
[2], ant column reconstruction retroperitoneally
[2], thoracotomy [11], or thoracophrenicolumbotomy
[3]) |
| outcomes |
- primary outcomes: donor site pain (at 6-wk, 3-mo,
6-mo, & 12-mo FU) measured w/ questionnaire &
localized tenderness
- secondary outcomes: functional disability during routine
activities or sleeping on op side, cosmesis,
plain x-ray appearance, clinical complications (all
determined blinded)
|
| loss to FU |
2 pts from each group |
| funding/COI |
none |
TABLE 9: Characteristics of Epstein study
| Characteristic |
Data |
| methods |
NRCT; duration 2–3 yrs
allocation: first 23 pts no reconstruction, second 23 pts
reconstruction |
| participants |
- mean age 44 yrs (range 23–67 yrs)
- eligibility criteria: 1-level ant cervical corpectomy & fusion,
w/ iliac crest harvest
- exclusion criteria: not stated
- total recruited: 46 pts (masking not stated); 28M:18F
|
| intervention |
MacroPore sheet & ICM; 23 pts |
| comparison |
no iliac crest reconstruction, bone wax hemostatic
agent; 23 pts |
| outcomes |
- primary outcomes: bodily pain (1 wk preop, & 6 wks, 3
mos, 6 mos, & 12 mos postop) measured using
SF-36
- secondary outcomes: CT appearance (masking not
stated)
|
| loss to FU |
none |
| funding/COI |
no declaration |
Bapat et al.4 reported that 15% of patients with versus
69% of those without reconstruction had donor site pain
at the 1-year follow-up (p = 0.001), with patients who had
undergone reconstruction also experiencing significantly
lower intensity of pain (p < 0.001). Tenderness on palpation
was elicited in a similar percentage of patients (p =
0.003).
Epstein and Hollingsworth12 commented that postoperative
pain measured through SF-36 bodily pain scores
revealed comparable results over the course of 12 months,
with a trend toward greater improvement in the group
without reconstruction. However, no statistical analysis
was performed.
Secondary Outcomes
Quality of Life and Functional Disability. Yang et al.33
examined comfort when wearing a belt with activity at 1
year, asking patients to categorize according to uncomfortable,
moderately comfortable, and comfortable. More
patients who underwent reconstruction reported being
comfortable, and more patients without reconstruction
reported being uncomfortable (p < 0.05).
Bojescul et al.5 reported that no patients from either
group had functional impairment from donor site pain at
1-year follow-up. Bapat et al.4 found that patients without
reconstruction experienced donor site pain causing
discomfort while sleeping on the operative side (31%),
discomfort wearing trousers (18%), and a persistent limp
(6%) at 1 year. No patients in the group with reconstruction
reported these functional disabilities.
Epstein and Hollingsworth12 provided data on a range
of health and function parameters as measured through
SF-36 data. Outcomes appeared similar; however, no statistical
analyses were performed.
TABLE 10: Methodological assessment of included NRCTs*
| Criteria (max score) |
Bapat et al. |
Epstein & Hollingsworth |
| reporting (10) |
9 |
8 |
| external validity (3) |
2 |
1 |
| internal validity–bias (7) |
6 |
4 |
| internal validity–confounding (6) |
1 |
1 |
| power (1) |
0 |
0 |
| total (27) |
18 |
14 |
* According to the validated checklist developed by Downs and Black.
Cosmesis. Yang et al.33 asked patients to categorize
their level of satisfaction with donor site cosmesis at 1
year as unsatisfied, moderately satisfied, or satisfied. All
25 patients who underwent reconstruction reported being
moderately satisfied or satisfied, whereas 12 of the 29
patients without reconstruction reported being unsatisfied
(no statistical analysis). The authors noted that a number
of patients in the nonreconstructed but not in the reconstructed
group exhibited clear surface indentation at the
harvest site.
Resnick25 determined that there was no significant
difference in unblinded surgeon–determined cosmesis
at 3 months. Bapat et al.4 reported significantly poorer
cosmetic VAS scores for their nonreconstructed group
as determined by a blinded observer (p = 0.009), with a
significantly higher number of Grades 2 and 3 iliac crest
defects (p < 0.001).
Radiological Analysis. Yang et al.33 found no graft
displacement on x-ray studies obtained at 1 year for their
rib-reconstructed group. Resnick25 reported that x-ray
evaluation of graft defects was not a useful method of
assessment.
Bojescul et al.5 found that 3 of 4 patients who underwent
CHA reconstruction (1 was lost to follow-up) had
evidence of bony ingrowth on x-ray and CT studies at
1 year. All had biological activity on bone scans. This
compared with 1 of 6 patients without reconstruction who
had bony ingrowth on x-ray and CT studies (p = 0.190),
and no patients with biological activity on bone scans (p
= 0.0048).
Bapat et al.4 reported that 19 of 20 rib grafts achieved
fusion at 6 months, evaluated primarily by x-ray studies.
One patient experienced graft resorption, and another
experienced graft displacement, whereas a third patient
suffered an iliac crest fracture intraoperatively during
graft impaction, and was reassigned to the nonintervention
group.
Epstein and Hollingsworth12 reported 100% fusion at
6 months in their reconstructed group as determined on
CT studies. Ectopic bone formation was observed to be
severe in 9%, moderate in 35%, and mild in 56% of cases,
but did not adversely affect outcome.
Resource Use. Only Epstein and Hollingsworth12 reported
results for this outcome, despite it not being mentioned
in the protocol. Hospital stay for the reconstructed
group was 3.6 days, compared with 3.2 days for the non-reconstructed group, leading the authors to conclude thatno difference existed (no statistical analysis). The baseline
operating time of 3 hours was increased by a mean of
24 minutes in the reconstructed group.
Postoperative Complications. Yang et al.33 reported
no complications for both groups. Resnick25 reported on
1 patient from the nonreconstructed group who experienced
a graft site infection requiring suture removal and
oral antibiotics. Bojescul et al.5 reported 1 superficial
infection of the harvest site in the reconstructed group,
which did not involve the implant. Bapat et al.4 reported
5 complications (31%) in 16 cases without reconstruction.
The complications were skin tenting and pressure necrosis,
bursitis, scar hypertrophy, infection, and persistent
limp. There were no complications in the reconstructed
group.
Discussion
Summary of Evidence
As far as we are aware, this review is the first attempt
to evaluate systematically the evidence for reconstruction
following iliac crest harvesting for spinal procedures.
Currently, there is insufficient high-quality evidence to
determine definitively the clinical utility of donor site
reconstruction following harvest for spinal procedures.
However, the best available data identified through this
systematic review suggest that iliac crest reconstruction
may be useful in reducing postoperative pain, minimizing
functional disability, and improving cosmesis. No pattern
of other clinical, radiological, or resource outcomes was
identified.
Limitations of This Review
The strength of any review relies on the quality of
the studies it examines. In this systematic review we identified
5 studies comparing reconstructive against no reconstructive
intervention for iatrogenic iliac crest defects.
Three studies were RCTs (totaling 96 patients) and 2 were
NRCTs (totaling 82 patients). All examined iliac crest
harvesting in the context of spinal fusion procedures.
Critical evaluation of the included studies revealed a
moderate to high risk of bias, especially in the NRCTs,
which were particularly prone to poor internal validity,
with a high risk of selection bias and confounding. Although
we decided to include these latter studies due to
the anticipated small number of RCTs, the specific limitations
of these NRCTs should be borne in mind when
evaluating their results.
Additional limitations include heterogeneity between
studies, such as patient population, level of spine operated
on (and hence size of defect), site of iliac crest grafting
(anterior vs posterior harvesting), harvesting technique,
reconstruction technique and type of graft used, method
of outcome evaluation, and the small number of patients
involved. As a result, specific questions such as harvesting
or reconstruction technique could not be evaluated.
Although we broadened our search strategy by not
applying any language restrictions, performing extensive
cross-referencing of relevant studies, and a search for recently completed or ongoing trials, our review is susceptible
to publication bias because we did not include an
extensive search for so-called gray literature. Studies that
did not show an effect for reconstruction would be most
likely to remain unpublished.
Conclusions
The evidence supporting reconstruction following
iliac crest harvest for spinal fusion is poor. However,
the best available evidence identified in this systematic
review supports the notion that iliac crest reconstruction
following harvest for spinal fusion may reduce postoperative
pain, minimize functional disability, and improve
cosmesis. The optimal type of graft material or surgical
technique was not investigated, and remains a relevant
question for future clinical studies.
Disclosure
The authors report no conflict of interest or sources of funding.
Author contributions to the study and manuscript preparation
include the following. Conception and design: Chau. Acquisition
of data: Chau, Xu. Analysis and interpretation of data: Chau, Xu,
Gragnaniello. Drafting the article: Chau, Xu, Van Der Rijt. Critically
revising the article: all authors. Reviewed submitted version of
manuscript: all authors. Approved the final version of the manuscript
on behalf of all authors: Chau. Administrative/technical/material
support:
Chau, Xu, Gragnaniello. Study supervision: Chau, Van Der
Rijt, Wong, Gragnaniello, Stanford, Mobbs.
References
- Acharya NK, Mahajan CV, Kumar RJ, Varma HK, Menon
VK: Can iliac crest reconstruction reduce donor site morbidity?:
a study using degradable hydroxyapatite-bioactive glass
ceramic composite. J Spinal Disord Tech 23:266–271, 2010
- Ahlmann E, Patzakis M, Roidis N, Shepherd L, Holtom P:
Comparison of anterior and posterior iliac crest bone grafts
in terms of harvest-site morbidity and functional outcomes. J
Bone Joint Surg Am 84-A:716–720, 2002
- Asano S, Kaneda K, Satoh S, Abumi K, Hashimoto T, Fujiya
M: Reconstruction of an iliac crest defect with a bioactive ceramic
prosthesis. Eur Spine J 3:39–44, 1994
- Bapat MR, Chaudhary K, Garg H, Laheri V: Reconstruction
of large iliac crest defects after graft harvest using autogenous
rib graft: a prospective controlled study. Spine 33:2570–2575,
2008
- Bojescul JA, Polly DW Jr, Kuklo TR, Allen TW, Wieand KE:
Backfill for iliac-crest donor sites: a prospective, randomized
study of coralline hydroxyapatite. Am J Orthop 34:377–382,
2005
- Chau AMT, Mobbs RJ: Bone graft substitutes in anterior cervical
discectomy and fusion. Eur Spine J 18:449–464, 2009
- Dave BR, Modi HN, Gupta A, Nanda A: Reconstruction of
iliac crest with rib to prevent donor site complications: a prospective
study of 26 cases. Indian J Orthop 41:180–182,
2007
- Defino HL, Rodriguez-Fuentes AE: Reconstruction of anterior
iliac crest bone graft donor sites: presentation of a surgical
technique. Eur Spine J 8:491–494, 1999
- Delawi D, Dhert WJA, Castelein RM, Verbout AJ, Oner FC:
The incidence of donor site pain after bone graft harvesting
from the posterior iliac crest may be overestimated: a study on
spine fracture patients. Spine 32:1865–1868, 2007
- Downs SH, Black N: The feasibility of creating a checklist for the assessment of the methodological quality both of randomised
and non-randomised studies of health care interventions.
J Epidemiol Community Health 52:377–384, 1998
- Dusseldorp JR, Mobbs RJ: Iliac crest reconstruction to reduce
donor-site morbidity: technical note. Eur Spine J 18:1386–
1390, 2009
- Epstein NE, Hollingsworth R: Does donor site reconstruction
following anterior cervical surgery diminish postoperative
pain? J Spinal Disord Tech 16:20–26, 2003
- Gil-Albarova J, Gil-Albarova R: Donor site reconstruction in
iliac crest tricortical bone graft: surgical technique. Injury
[epub ahead of print], 2011
- Hardy JH: Iliac crest reconstruction following full-thickness
graft: a preliminary note. Clin Orthop Relat Res (123):32–
33, 1977
- Harris MB, Davis J, Gertzbein SD: Iliac crest reconstruction
after tricortical graft harvesting. J Spinal Disord 7:216–221,
1994
- Higgins JPT, Green S (eds): Cochrane Handbook for Systematic
Reviews of Interventions, Version 5.1.0 [updated
March 2011]. The Cochrane Collaboration, 2011 (http://www.
cochrane-handbook.org/) [Accessed March 13, 2012]
- Hochschuler SH, Guyer RD, Stith WJ, Ohnmeiss DD, Rashbaum
RF, Johnson RG: Proplast reconstruction of iliac crest
defects. Spine 13:378–379, 1988
- Howard JM, Glassman SD, Carreon LY: Posterior iliac crest
pain after posterolateral fusion with or without iliac crest
graft harvest. Spine J 11:534–537, 2011
- Ito M, Abumi K, Moridaira H, Shono Y, Kotani Y, Minami
A, et al: Iliac crest reconstruction with a bioactive ceramic
spacer. Eur Spine J 14:99–102, 2005
- Kim DH, Rhim R, Li L, Martha J, Swaim BH, Banco RJ, et
al: Prospective study of iliac crest bone graft harvest site pain
and morbidity. Spine J 9:886–892, 2009
- Lubicky JP, DeWald RL: Methylmethacrylate reconstruction
of large iliac crest bone graft donor sites. Clin Orthop Relat
Res 164:252–256, 1982
- Morgan SJ, Jeray KJ, Saliman LH, Miller HJ, Williams AE,
Tanner SL, et al: Continuous infusion of local anesthetic at
iliac crest bone-graft sites for postoperative pain relief. A
randomized, double-blind study. J Bone Joint Surg Am 88:
2606–2612, 2006
- National Health and Medical Research Council: NHMRC
Levels of Evidence and Grades for Recommendations
for Developers of Guidelines, December 2009. Australian
Government National Health and Medical Research Council,
2009 (http://www.nhmrc.gov.au/_files_nhmrc/file/guidelines/
evidence_statement_form.pdf) [Accessed March 13, 2012]
- Pollock R, Alcelik I, Bhatia C, Chuter G, Lingutla K, Budithi
C, et al: Donor site morbidity following iliac crest bone harvesting cervical fusion: a comparison between minimally
invasive and open techniques. Eur Spine J 17:845–852, 2008
- Resnick DK: Reconstruction of anterior iliac crest after bone
graft harvest decreases pain: a randomized, controlled clinical
trial. Neurosurgery 57:526–529, 2005
- Schwartz CE, Martha JF, Kowalski P, Wang DA, Bode R, Li
L, et al: Prospective evaluation of chronic pain associated
with posterior autologous iliac crest bone graft harvest and
its effect on postoperative outcome. Health Qual Life Outcomes
7:49, 2009
- Sengupta DK, Truumees E, Patel CK, Kazmierczak C, Hughes
B, Elders G, et al: Outcome of local bone versus autogenous
iliac crest bone graft in the instrumented posterolateral fusion
of the lumbar spine. Spine 31:985–991, 2006
- Silber JS, Anderson DG, Daffner SD, Brislin BT, Leland JM,
Hilibrand AS, et al: Donor site morbidity after anterior iliac
crest bone harvest for single-level anterior cervical discectomy
and fusion. Spine 28:134–139, 2003
- Singh K, Phillips FM, Kuo E, Campbell M: A prospective,
randomized, double-blind study of the efficacy of postoperative
continuous local anesthetic infusion at the iliac crest bone
graft site after posterior spinal arthrodesis: a minimum of
4-year follow-up. Spine 32:2790–2796, 2007
- Tanishima T, Yoshimasu N, Ogai M: A technique for prevention
of donor site pain associated with harvesting iliac bone
grafts. Surg Neurol 44:131–132, 1995
- Wai EK, Sathiaseelan S, O'Neil J, Simchison BL: Local administration
of morphine for analgesia after autogenous anterior
or posterior iliac crest bone graft harvest for spinal fusion:
a prospective, randomized, double-blind, placebo-controlled
study. Anesth Analg 110:928–933, 2010
- Wang MY, Levi ADO, Shah S, Green BA: Polylactic acid
mesh reconstruction of the anterior iliac crest after bone harvesting
reduces early postoperative pain after anterior cervical
fusion surgery. Neurosurgery 51:413–416, 2002
- Yang Y, Chen X, Zan Z, Tang H, Huang Y, Tang L, et al:
[Clinical application of rib autograft for iliac crest reconstruction
by anterior approach of thoracic and lumbar vertebrae.]
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 23:1334–
1337, 2009 (Chinese)
Manuscript submitted November 17, 2011.
Accepted March 12, 2012.
Please include this information when citing this paper: published
online April 13, 2012; DOI: 10.3171/2012.3.SPINE11979.
Address correspondence to: Ralph J. Mobbs, M.B.B.S., M.S.,
F.R.A.C.S., Suite 7a, Level 7, Prince of Wales Private Hospital,
Randwick, New South Wales 2031, Australia. email: ralphmobbs@hotmail.com.
 Reconstruction iliac crest defects following harvest for spinal fusion: a systematic review Reconstruction iliac crest defects following harvest for spinal fusion: a systematic review
You will need the Adobe Reader to view and print the above documents. 
|
