|
Technique, challenges and indications for percutaneous pedicle screw fixation
Ralph J. Mobbs a,*, Praveenan Sivabalan b, Jane Li b
- Department of Neurosurgery, Prince of Wales Private Hospital, Suite 3, Level 7, Sydney Spine Clinic, Randwick, New South Wales 2031, Australia
- Clinical School, Faculty of Medicine, University of New South Wales, Sydney, New South Wales, Australia
Abstract
Minimally invasive techniques in spinal surgery are increasing in popularity due to numerous potential
advantages, including reduced length of stay, blood loss and requirements for post-operative analgesia as
well as earlier return to work. This review discusses guidelines for safe implantation of percutaneous
pedicle screws using an image intensifier technique. As indications for percutaneous pedicle screw techniques
expand, the nuances of the minimally invasive surgery technique will also expand. It is paramount
that experienced surgeons share their collective knowledge to assist surgeons at their early attempts of
these complex, and potentially dangerous, procedures. Technical challenges of percutaneous pedicle
screw fixation techniques are also discussed including: small pedicle cannulation, percutaneous rod
insertion for multilevel constructs, incision selection for multilevel constructs, changing direction with
percutaneous pedicle screw placement, L5/S1 screw head proximity and sclerotic pedicles with difficult
Jamshidi placement. We discuss potential indications for minimally invasive fusion techniques for complex
spinal surgery and support these with descriptions of illustrative patients.
Crown Copyright © 2010 Published by Elsevier Ltd. All rights reserved.
1. Introduction
Pedicle screw instrumentation enables a rigid construct to promote
stability and fusion for numerous spinal pathologies including:
trauma, tumours, deformity and degenerative disease. The
safety of traditional open techniques for pedicle screw placement
has been well documented; however, due to the advantages of
minimally invasive surgery (MIS), demand for percutaneous pedicle
screw insertion will increase. Improvements in minimally invasive
instrumentation have also broadened the scope of spinal
disorders that surgeons can operate on.1,2
Percutaneous pedicle screw insertion can be an intimidating
prospect for surgeons who have been trained in open techniques
only. The initial change has a steep learning curve; however, there
are several basic principles that can assist the surgeon in safe
placement of the Jamshidi needle into a thoracic or lumbar pedicle.
2. Open versus minimally invasive surgery
Conventional open spine surgery has several reported limitations
including extensive blood loss, post-operative muscle pain
and infection risk. The paraspinal muscle dissection involved in
open spine surgery (Fig. 1) can cause muscular denervation, increased
intramuscular pressure, ischaemia, necrosis and revascularisation injury resulting in muscle atrophy and scarring, often
associated with prolonged post-operative pain and disability.
Spinal fixation utilising muscle-dilating approaches (Figs. 1 and
2) to minimise surgical incision length, surgical cavity size and
the amount of iatrogenic soft-tissue injury associated with surgical
spinal exposure, without compromising outcomes, is thus a desirable
advancement.3–12
No published articles of high-quality show that MIS is superior
to open spinal surgery; however, there is a trend towards MIS of
the spine due to lower complication rates and approach-related
morbidity, with minimal soft tissue trauma, reduced intra-operative
blood loss/risk of transfusion, improved cosmesis, decreased
post-operative pain and narcotic usage, shorter hospital stays with
faster return to work and thus reduced overall health care
costs.1,4,6–9,13,14 Despite this, some reports believe that minimal
exposure is associated with incomplete treatment of pathology,13
due to significantly decreased visualisation with MIS.10 Another
potential limitation includes the use of imaging-guided pedicle
screw placement. Imaging increases operating times and patient/
surgeon exposure to ionising radiation. Non-radiological navigation
methods thus need to be explored to further improve MIS.3,10
The senior author (RJ Mobbs) has inserted more than 700 percutaneous
pedicle screws (Fig. 2) with two significantly misplaced
screws (0.29%), and one screw placement resulting in a permanent
nerve root injury with a pedicle fracture (0.14%). Both complications
were within the initial 10 patients, representing a steep
learning curve with this technique.
3. Percutaneous placement of pedicle screws
The technique described here uses intra-operative radiography
(image intensifier [II]). The senior author also uses intra-operative
CT-based stereotactic guidance for pedicle screw placement; however,
there is a greater degree of accuracy with the use of II for pedicle
cannulation (RJ Mobbs, unpublished data). For small thoracic
pedicles, the senior author only uses II due to the enhanced accuracy
with this technique.
The sequence of percutaneous placement of pedicle screw
insertion is described as follows (Figs. 2 and 3).

Fig. 1. Open versus (vs.) minimally invasive surgery (MIS) technique for pedicle
screw insertion showing: (a) normal anatomy; (b) muscle retraction with
traditional ''open'' surgery vs. (c) ''tubular retractors'' with percutaneous pedicle
screws.
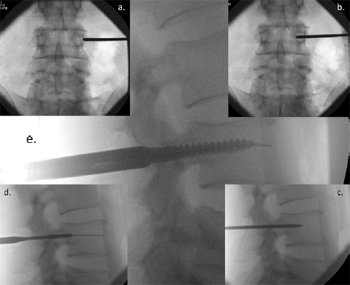
Fig. 2. Image Intensifier radiographs of the percutaneous technique for pedicle screw
insertion showing: (a) anterior/posterior (AP) view of the Jamshidi needle docked
onto the lateral aspect of the pedicle – the ''3 o'clock position''; (b) AP view of
advancement of the needle 20 mmto 25 mminto the vertebral body; (c) lateral view,
checking the position of the Jamshidi needle in lateral view; (d) lateral view, the Kwire
and tapping of the pedicle; and (e) lateral view, insertion of the pedicle screw.

Fig. 3. Diagrams illustrating the anatomical principles of percutaneous pedicle
screw insertion: views from top to bottom: (a) superior, (b) posterior, (c) lateral, (d)
superior. First the initial skin incision is made with the patients' body habitus in
mind. Second, the Jamshidi needle is first ''docked'' onto the lateral aspect of the
pedicle – ''position 1'' – on the anterior/posterior image intensifier (II) radiograph
projection. Third, the Jamshidi needle is advanced 20 mm to 25 mm so that the
needle is beyond the medial border of the pedicle and into the vertebral body – to
''position 3''. Finally, the position is confirmed by lateral II radiograph projection
before insertion of the K-wire.
- Place the II in the anterior/posterior (AP) position. The spinous
process should be midline between the pedicles to
ensure a direct AP projection (Fig. 2a).
- Mark the position of the lateral aspect of the pedicle on the
skin. Depending upon the depth of the tissue between skin
and pedicle, the skin incision should be made laterally
(Fig. 3) so that the Jamshidi needle can be angled appropriately
when inserting it into the pedicle.
- Place the Jamshidi needle through the skin incision and
''dock'' onto the lateral aspect of the pedicle (Fig. 2a). This
is called the ''3 o-clock'' position.
- Advance the Jamshidi needle 20 mm to 25 mm into the pedicle,
making sure the needle remains lateral to the medial
pedicle wall (Figs. 2b and 3).
- Position the II in the lateral plane. The Jamshidi needle
should now be in the vertebral body, and therefore ''safe''
with no risk of medial pedicle breach (Fig. 2c).
- Place a K-wire down the Jamshidi needle and place a pedicle
tap down the trajectory of the K-wire (Fig. 2d).
- Place the final pedicle screw with the screw placed down the
K-wire (Fig. 2e), making sure not to advance the K-wire
beyond the anterior aspect of the vertebral body.
4. Challenges unique to MIS/percutaneous pedicle screw
insertion
There are many technical challenges unique to the percutaneous
pedicle screw insertion technique. After the surgeon is comfortable
with MIS techniques for single-level degenerative
pathologies, the temptation is to attempt more difficult multi-level
constructs such as those required in patients with tumour and
trauma pathologies.
The senior author has identified common challenges described in the following sections.
4.1. Changing direction of screw placement following initial pedicle cannulation
Following initial placement of the Jamshidi needle into the vertebral
body, the lateral projection may demonstrate a poor trajectory
that the surgeon may wish to correct. Using the pedicle tap
placed down the K-wire, the surgeon can ''redirect'' the tap along
a more appropriate trajectory, using an undersized tap (Fig. 4). This will result in an acute bend in the K-wire distal to the tap. Care
should be taken not to bend the K-wire too much (Fig. 4b). With
the tap advanced into the vertebral body, the K-wire can be removed
and then a fresh ''straight'' K-wire reintroduced down the
tap into the new position (Fig. 4c). The pedicle screw can then be
placed down the K-wire in the new trajectory.
4.2. L5/S1 screw head proximity
The percutaneous technique can be difficult at the L5/S1 level
due to the L5 and S1 pedicle angulations. The percutaneous retraction
sleeves can impinge on one another at skin level. The options
for dealing with this common problem include either placing the
S1 pedicle screw in a more inferior starting position, or to use
''flexible'' retraction sleeves (Fig. 5) so that the sleeves can be deflected
at skin level and not impinge on each other.
4.3. Cannulation of small pedicles
The senior author has placed percutaneous pedicle screws as
high as T4 in patients with tumour and trauma; however, percutaneous
pedicle placement high in the thoracic spine can be technically
difficult due to the small pedicle sizes in the mid-upper thoracic
spine and the change in pedicle angulation at the T1 to T4 levels.
The greatest challenge, however, for percutaneous placement in
the thoracic spine is from small pedicles. Cannulation of small pedicles
involves careful evaluation of the pre-operative CT scans and
and AP radiographs to ensure that the pedicle can be cannulated.
The pedicle must have a width of at least 3 mm to 4 mm so that
the Jamshidi needle can be navigated down the pedicle. The surgeon
must have an excellent view of the pedicle prior to attempting cannulation
of a small diameter pedicle. This involves small movements
of the gantry of the II machine so that the II is directed in a ''bull's
eye'' view down the pedicle (Fig. 6). Due to the greater degree of
accuracy of the II technique (Fig. 6) over stereotaxis, the senior
author recommends II for small pedicle cannulation.

Fig. 4. Image Intensifier lateral radiographs showing changing direction of screw
placement following initial pedicle cannulation. (a) The Jamshidi needle has
cannulated the pedicle and vertebral body. On review of the lateral X-ray image, the
surgeon decides to re-direct the screw into a position that is more parallel with the
endplate. (b) After K-wire insertion, an undersized tap can be used to force the Kwire
into a more appropriate direction, taking care to avoid bending the K-wire too
much. (c) With the tap in place, the K-wire can be removed and replaced with a
new, straight K-wire down the tap in the new direction.
4.4. Skin Incision selection for multi-segmental fixation
Multi-segmental fixation procedures have numerous nuances
that can make the surgeon's life much easier. The first thought is incision selection, as incisions that ''line-up'' in a straight line
(Fig. 7) represent a far easier prospect for rod insertion. The qualification
here is for patients who have a scoliosis, or trauma, where
the pedicles may not line up in a straight line. For a single-level fixation,
the rod insertion is not difficult and is usually not a problem.
For multi-level fixation, a staggered line of incision points can result
in a difficult rod insertion.
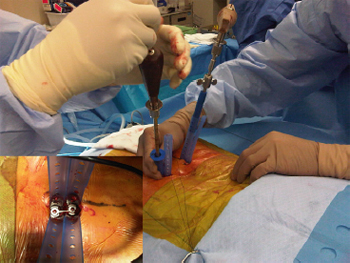
Fig. 5. Intra-operative photographs showing the use of retraction sleeves to avoid
screw head proximity at L5/S1. Flexible retraction sleeves simplify the problem of
screw head proximity as the retractors can easily be deflected and not ''get in your
way'' with percutaneous pedicle screw placement. It is common at L5/S1 for the
surgeon to require a single incision only as the retraction sleeves are in the same
position at the skin edge. (Insert) Aerial view.
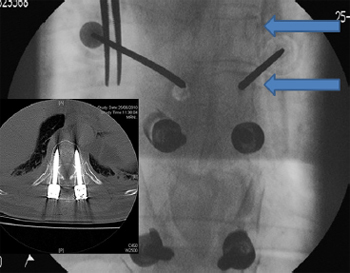
Fig. 6. Anterior/posterior image intensifier (II) radiographs showing percutaneous
cannulation of small pedicles. Angulating the II machine so that the surgeon is
looking down the pedicle – the ''bulls eye'' view (arrows) – assists in placement in
difficult pedicles. (Insert) The II technique results in highly accurate pedicle screw
insertion – a 5.5 mm screw into a 6 mm pedicle.

Fig. 7. Intra-operative photograph of skin incision for multi-level constructs. The
black line is showing all four incisions along a straight trajectory – making the
insertion of the rod technically much easier. The white line shows the incisions in a
staggered fashion – the rod insertion here will be more difficult.
4.5. Insertion of a rod for multi-segmental fixation
Rod insertion for multi-segmental fixation involves the surgeon
having a brief mental checklist prior to insertion. Removing the rod
after an initial placement can be difficult and time consuming if the
surgeon has to change the length or curvature of the rod prior to a
re-insertion. The checklist includes:
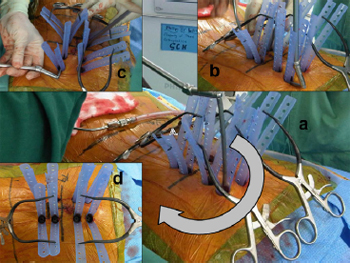
Fig. 8. Intra-operative photographs showing insertion of rod in a patient requiring
multi-level surgery. (a) Rod insertion can be performed with a semicircular
technique (arrows), making sure that the rod is under the fascial layer with (b, c)
advancing the rod. Always start the insertion at the pedicle screw head that is most
superficial/closest to the skin. (d) Aerial view.
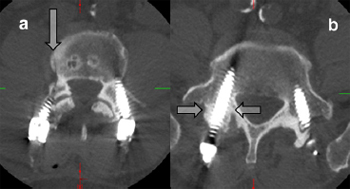
Fig. 9. Axial CT scans showing sclerotic pedicles that may result in the necessity for
screw placement using an open technique. (a) The arrow points towards a Jamshidi
needle tract created when the Jamshidi needle ''hit'' a sclerotic bar of bone between
the pedicle and vertebral body – safe cannulation of the pedicle/vertebral body
could not be achieved and a high-speed drill was used to cannulate the pedicle. (b)
This pedicle (arrows) was sclerotic – Jamshidi placement required an open screw
positioning.
- How long should the rod be? The length between the retraction
sleeves should provide a guide to rod length.
- Does the rod require bending before insertion? As a general
rule, the surgeon should try to leave the pedicle screw heads
at an equivalent height throughout the construct to aid with
ease of rod insertion. The exception here is with trauma
where a reduction manoeuvre may be required.
- Initially from which end should the rod be inserted? The rod
should be inserted from the end of the construct where the
pedicle screw head is closest to the skin, enabling ease of
insertion and navigation along the pedicle screw heads
(Fig. 8).
- Do I need an additional incision to insert the rod? This is
usually not necessary as the rod can be inserted over many
levels via the cranial incision which is usually the most
superficial of the pedicle screw insertions.
4.6. Sclerotic pedicle – difficult Jamshidi placement in hard pedicles
Pedicles that are sclerotic or osteopetrotic (''ivory bone'') can be
difficult in terms of Jamshidi placement. Advancing a Jamshidi into
these pedicles can be frustrating – rarely the percutaneous technique needs to be abandoned and an open technique with
direct cannulation of the pedicle with a high-speed drill is required
(Fig. 9).
5. Indications for percutaneous pedicle screw insertion
5.1. Degenerative spine disorders
Paraspinal muscle retraction allowing adequate exposure in
open procedures is a primary cause of post-laminectomy syndrome
and ''fusion disease''.11,12 Since the first endoscopic lumbar
discectomy in 1991, MIS has been used to routinely treat degenerative
spinal pathologies including; herniated disc removal, spinal
stenosis decompression and/or fusion aiming to avoid these problems.
Preliminary clinical outcomes suggest MIS is as efficacious as
open spinal surgery for degenerative spinal disorders, with added
advantages of reduced recovery times, pain and days to return to
work. However, as the use of MIS in complex spinal surgery is only
in its infancy, these favourable results are largely anecdotal and yet
to be validated by long-term outcome studies.4,11
MIS fusion is indicated for mechanical lower back-pain and
grade I and II spondylolisthesis-associated radicular pain
(Fig. 10). Higher grade spondylolistheses prove more challenging
and open approaches are recommended for optimal management.
14 Harris et al.'s3 comparison of 29 patients receiving single/
double level posterolateral percutaneous instrumented fusion
for symptomatic spondylolisthesis with published open fusion results
revealed comparable improvements in pain and disability,
whereas mean blood loss and operating time were significantly
lower with the use of MIS (222 mL vs. 1517 mL; 141 minutes vs.
298 minutes).3
MIS is also indicated for recurrent disc herniation, pseudoarthrosis
and severe discogenic lower back pain15 resulting from
post-laminectomy instability or spinal trauma.14
5.1.1. Illustrative patient 1
A 73-year-old male presented with neurogenic claudication and
mechanical lower back pain of 3 years' duration. Imaging revealed
severe canal stenosis at L4/5 due to spondylolisthesis, and facet joint/ligamentous hypertrophy. A midline incision and posterior
lumbar interbody fusion was performed. The midline incision was
closed and percutaneous screws inserted using the II technique.
Blood loss was 180 mL with discharge from hospital on day 4.

Fig. 10. Middle panel. Post-operative photograph of illustrative patient 1, a 73-
year-old male who presented with L4/5 degenerative spondylolisthesis with
neurogenic claudication. (a) Lateral image intensifier (II) radiograph showing Grade
I spondylolisthesis; (b) sagittal T2-weighted MRI showing severe canal stenosis; (c)
photograph at 8 weeks showing post-operative incision; (d) lateral II image
showing initial midline incision and posterior lumbar interbody fusion; (e) lateral II
percutaneous pedicle screw fixation pre-reduction; and (f) lateral II final radiograph
showing reduction of spondylolisthesis.

Fig. 11. Illustrative patient 2, a 17-year-old male who presented with a T12
American Spinal Injury Association (ASIA) – A spinal cord injury following a high
velocity motorcycle accident. (a) Sagittal T2-weighted MRI showing T12 spinal
injury with ASIA – A neurological deficit; (b) intra-operative photograph showing
percutaneous pedicle screw fixation; (c) anterior/posterior image intensifier (II)
radiograph and (d) lateral II radiograph showing stabilisation; and (e) postoperative
photograph showing early mobility within 24 hours.
5.2. Trauma
Traumatic spinal injuries are often associated with high velocity,
high energy impacts, (e.g. falls, motor vehicle crashes). Early
surgical intervention may prevent or potentially reverse neurological
deterioration.4 Surgical management involves decompression,
reduction, anterior column support if necessary, restoration of posterior
tension band and fusion to prevent spinal deformity developing
while providing immediate spinal stability.4,16 Current
surgical spine trauma treatment is predominantly open surgery
with instrumentation and fusion.16 Trauma patients, however,
are at greater risk of intra-operative blood loss with infection rates
of 0.7% to 10%. These vulnerabilities, along with other comorbidities
and the strong likelihood of systemic injuries in spinal trauma
patients, make MIS approaches highly valuable for minimising access-
related morbidity.4,16
Percutaneous instrumentation with/without fusion is performed
following thoracolumbar injuries for spinal stabilisation.4
Thoracic pedicle screw utilisation for degenerative and traumatic
injuries is one of the newest developments in MIS; however, morbidity
associated with screw misplacement in the thoracic spine is
greater than for the lumbar spine as there is greater risk of spinal
cord lesions, paraplegia, and fatal great vessel injury.13
Posterior MIS spinal fusion approaches such as posterior pedicle
screw/rod fixation are being applied to thoracic spine fracture
management (Fig. 11), providing stand-alone fixation of stable
burst or flexion distraction injuries. Temporary percutaneous posterior
fixation can enable mobilisation and prevent secondary injury
when there is an unstable injury and complete fixation is
contraindicated. Despite these developments, there are no established
MIS techniques in thoracic spine trauma surgery.16
5.2.1. Illustrative patient 2
A 17-year-old male presented with a T12 American Spinal
Injury Association–A spinal cord injury following a high velocity motorcycle accident. Stabilisation surgery was performed the day
of presentation with mobilisation and wheelchair rehabilitation
within 24 hours (Fig. 11). Surgical time was 2 hours, 5 minutes
with 80 mL of blood loss. The patient requested pedicle screw removal
12 months following surgery due to discomfort of the pedicle
screw tulips against his wheelchair.
5.3. Spinal neoplasia
Up to 70% of cancer patients show evidence of metastatic disease
at death, with 40% having spinal involvement.6 Improvements
in systemic cancer management and imaging is expected to increase
the incidence of spinal metastases detection.4 Metastatic
spine disease arises most commonly in the thoracic spine (thoracic
70%; lumbar 20%; cervical 10%) with 10% to 20% suffering symptomatic
cord compression causing neurological dysfunction and
debilitating pain requiring treatment.4,6
Studies on metastatic spinal disease show better functional outcomes
following surgical decompression/stabilisation prior to radiation
than radiation alone (84% vs. 57%). Surgery prolongs survival,
maintains continence and reduces corticosteroids and analgesic
use.6 Oncology patients often suffer multiple comorbidities, so efforts
to reduce surgical morbidity are essential.16 Although treatment
is often palliative, it is crucial in improving quality of life
(QOL) by improving pain and ambulatory function.4 Thus, the
advantages of MIS, including smaller incisions limiting wound
complications, are crucial for maintaining/improving the QOL of
cancer patients with a mean survival of only 8 to 12 months.4,6
Another recently introduced stabilisation technique for spinal
neoplasia utilises percutaneous instrumentation with cement
reconstruction and/or placement of intervertebral structural grafts.
The combination of increasingly available MIS management options
(Fig. 12), and chemo/radiotherapy will likely improve spinal
cancer treatment.4
5.3.1. Illustrative patient 3
A 69-year-old male presented with progressive paraparesis and
cord compression at T9. He had metastatic lung cancer with an expected
longevity of less than 12 months. MIS decompression was
proposed; however, stabilisation was recommended due to the
anterior compression and pediculectomy/partial vertebrectomy
necessary for adequate tumour resection. Surgical time was 2
hours and 35 minutes with 210 mL of blood loss and a length of stay (LOS) of 5 days. The patient remained independently mobile
until his death 7 months following surgery.
5.4. Infection
Vertebral osteomyelitis is relatively uncommon, accounting for
3% of total osteomyelitis; however, its incidence worldwide is
growing and it causes substantial morbidity.17,18 Osteomyelitis of
the thoracic spine causes vertebral body collapse and thus spinal
cord compromise or kyphosis.16 Surgical indications exist,
although most treatment is conservative, utilising antibiotics.
These indications include the need for bacterial diagnosis when
other methods fail, abscess drainage, decompression of neural elements
causing worsening neurological deficit, debridement of persisting
infection and restoration and/or maintenance of spinal
alignment and stability.16–18
Current surgical management of osteomyelitis includes thoracotomy,
corpectomy and reconstruction. Open anterior thoracic
spine exposure for vertebral osteomyelitis is associated with high
mortality,16,17 partly due to the frequent occurrence of vertebral
osteomyelitis in the elderly, the debilitated and patients with multiple
comorbidities. Thus MIS can potentially improve outcomes
(Fig. 13).17
A small study of patients with pyogenic vertebral osteomyelitis,
who were treated with thoracoscopic debridement, decompression
and anterior fusion with no disease recurrence after two years,
suggested the feasibility of MIS for vertebral osteomyelitis.17 Larger
studies are required to further deduce whether MIS is beneficial
as only small MIS studies for vertebral osteomyelitis with
short-term follow-up exist.17
5.4.1. Illustrative patient 4
A 47-year-old woman positive for hepatitis C and human immunodeficiency
virus presented with L1/L2 osteomyelitis. The patient
had developed progressive pain and leg weakness over 2 months
with vertebral body collapse and gross mechanical instability at
L1/L2. Open surgery was not offered due to her pre-morbid status
and high-risk to the surgical team. Percutaneous stabilisation of
the progressive kyphosis/vertebral body collapse was offered. Surgical
time was 1 hour, 55 minutes with 120 mL of blood loss. The patient
was mobilised on day 1 with significantly reduced pain scores
and discharged to the infectious diseases team. Follow-up at 4
months revealed bone union across the L1/L2 interspace.
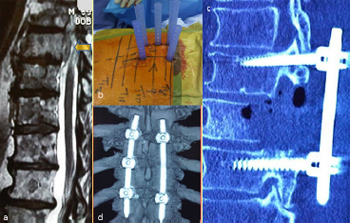
Fig. 12. Illustrative patient 2, a 69-year-old male who presented with progressive
paraparesis and cord compression at T9. (a) Sagittal T2-weighted MRI showing T9
cord compression from lung metastasis (arrow); (b) intra-operative photograph
showing percutaneous screw fixation; (c) sagittal post-operative CT scan reconstruction
showing partial vertebrectomy and decompression; and (d) post-operative
three-dimensional CT scan reconstruction showing stabilisation.
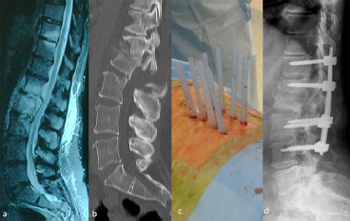
Fig. 13. Illustrative patient 4, a 47-year-old female, positive for hepatitis C and
human immunodeficiency virus, with L1/L2 osteomyelitis. (a) Sagittal T2-weighted
MRI showing L1/L2 osteomyelitis; (b) sagittal CT scan reconstruction showing
progressive kyphosis; (c) intra-operative photograph showing percutaneous pedicle
screw fixation; and (d) post-operative lateral image intensifier radiograph showing
a reduction of deformity and restoration of sagittal alignment.
5.5. Obesity
All spinal operations prove more difficult in patients with obesity,
9 and they have increased complication risks including surgical
site infection following fusion. However, MIS posterior lumbar fusion
is especially useful for these patients.13,19 Open posterior lumbar
fusions require longer incisions to access the deeper spine in
obese patients.9,19 Tubular retraction systems used in MIS, however,
enable the use of similarly sized incisions for all patients.
Shorter incisions minimise surgical cavity size, reduce soft-tissue
trauma, and produce an instrument-only surgical field, reducing
complications experienced by obese patients, including wound
infections.9,19 Excessive body weight also requires longer operating
times for open posterior lumbar spine fusion, but no significant difference
in operative times for MIS techniques, as the greater skin to
spine distance does not require additional dissection time when
using minimally invasive tubular retractor systems.19 These advantages
indicate the use of MIS in obese patients (Fig. 14).
5.5.1. Illustrative patient 5
A 59-year-old male presented with mechanical back pain, unilateral
L5 radiculopathy due to lateral recess stenosis and an elevated
body mass index. MIS–transforaminal lumbar interbody
fusion (TLIF) was recommended to avoid a lengthy incision and
prolonged hospital stay. Surgical time was 4 hours and 50 minutes
with 240 mL of blood loss. LOS was 3 days. Frameless stereotaxis
was used to assist with percutaneous pedicle screw placement.
5.6. Revision surgery
Revision surgery is often more technically challenging because
of local scarring and greater complication rates such as nerve root
injury and incidental durotomy. Along with altered anatomy, absent
bony landmarks and limited surgical exposure, it is no surprise
that surgeons avoid MIS approaches for revision surgery.8,9
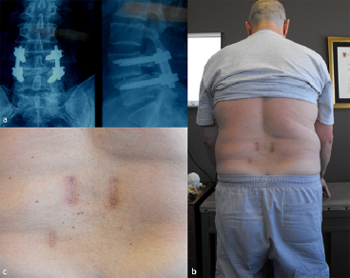
Fig. 14. Illustrative patient 5, 59-year-old, 135 kg male, who presented with
mechanical back pain, unilateral L5 radiculopathy due to lateral recess stenosis and
an elevated body mass index who underwent a transforaminal lumbar interbody
fusion (TLIF). (a) Left – anterior/posterior (AP) image intensifier (II) radiograph, and
right – lateral II radiograph showing L4/5 TLIF plus pedicle screw fixation; (b) 6-
month post-operative photograph of the patient standing; (c) close-up of 4 cm
bilateral incision, plus bone graft harvest, scars.
Lumbar interbody fusions are indicated for revision surgery of
recurrent disc herniation and post-laminectomy instability. Selznick
et al.'s study8 of 43 patients who underwent minimally invasive
posterior lumbar interbody fusion (PLIF) or TLIF, compared the outcomes following primary surgery to revision surgery at a prior
operative level. The primary surgery group consisted of 26 patients
undergoing operations for degenerative spondylolisthesis and
spondylolysis, degenerative scoliosis. The revision surgery group
consisted of 17 patients with the primary indications for the surgery
being post-laminectomy instability and multiple recurrent
disc herniations. They concluded that minimally invasive lumbar
interbody fusion is a possible option for revision surgery, without
significantly higher rates of blood loss, transfusion, infection or
neurological complications compared to primary surgery. However,
minimally invasive revision lumbar interbody fusions had
significantly higher complication rates, with the risk of inadvertent
durotomy and cerebrospinal fluid (CSF) leak approximately six
times higher than in the primary surgery cohort. All CSF leaks were
fixed intra-operatively without developing into a pseudomeningocele
or requiring further surgery.8
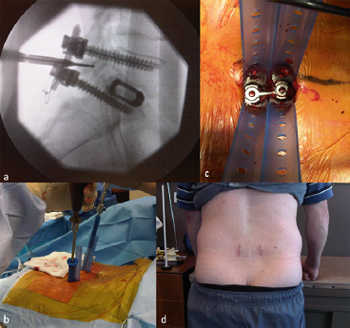
Fig. 15. Illustrative patient 6, a 48-year-old male who presented with ongoing back
pain and evidence of a non-union following a L5/S1 stand-alone posterior lumbar
interbody fusion (PLIF). (a) Lateral image intensifier (II) intra-operative radiograph
showing pedicle screw fixation and posterolateral graft. (b, c) Intra-operative
photographs showing completion of procedure prior to removal of tubular dilators.
(d) Post-operative photograph at 10 weeks showing previous midline incision and
bilateral revision fixation using minimally invasive surgery.
It is recommended that surgeons gain substantial experience
with MIS techniques of primary patients, before attempting minimally
invasive revision interbody fusion of the lumbar spine.8,9
Surgeons attempting minimally invasive revision surgery
(Fig. 15) should also be ready to convert to wider exposures if necessary,
for safe exposure of the relevant spinal region.9
5.6.1. Illustrative patient 6
A 48-year-old male presented with ongoing back pain and evidence
of a non-union following a L5/S1 stand-alone PLIF. Revision
fusion with percutaneous pedicle screws and on-lay bone graft
over the L5/S1 facet joints was recommended. Operating time
was 1 hour, 40 minutes with 100 mL of blood loss. LOS was 3 days.
Significant immediate reduction in mechanical back pain was
experienced, with return to work within 6 weeks.
5.7. MIS grafting
Autografts involve transferring bone within the same individual.
20 Throughout the 1990s, as spinal fusion rates rose, bone graft harvests were most commonly used for spinal arthrodesis.21,22
With fusion, the gaps between host bone and graft fill via new bone
formation and with more bone deposition and remodelling on the
osteoconductive matrix, segmental stiffness increases. Thus, spinal
bone grafting is a race between fusion healing and failure of internal
fixation to immobilise spinal elements.20
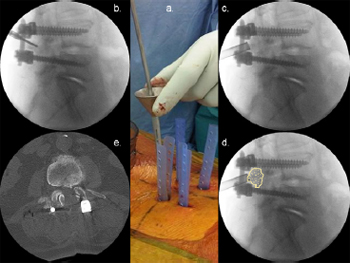
Fig. 16. Illustrative patient 7, a 72-year-old female who presented with neurogenic
claudication and L4/5 grade 1 spondylolisthesis. (a) Intra-operative photograph
showing the minimally invasive surgery grafting technique for a posterolateral
graft. (b, c, d) Lateral intra-operative image intensifier radiographs showing (b) drill
preparation of facet joints; (c) insertion of graft packing tube; and (d) position of
posterolateral graft. (e) Axial post-operative CT scan with graft overlay on facet
joint.
An adequate blood supply is necessary to encourage healing.
Excessive muscle stripping and devascularisation limits oxygen,
nutrient, neovascularisation and cellular migration to the fusion
mass. Significant muscle necrosis may also provide environments
suitable for bacterial growth, which compete for nutrients and
interfere with the inflammatory processes necessary for the developing
fusion mass, thus causing graft failure.20 MIS, which aims to
minimise blood loss, muscle stripping and necrosis thus promotes
successful fusion (Fig. 16). With the limited number of studies
available, however, it is suggested that evidence based medicine
guide the use of bone grafts and bone morphogenic protein in minimally
invasive spinal fusion.13
5.7.1. Illustrative patient 7
A 72-year-old woman presented with neurogenic claudication
and L4/5 grade 1 spondylolisthesis. Decompression and posterolateral
onlay fusion was recommended. Interbody fusion was not
recommended due to poor bone mineral density. Following a midline
MIS decompression, percutaneous pedicle screws were inserted
and postero-lateral bone graft onlay was performed via
the retraction tubes for the pedicle screw system.
5.8. Emerging technology
Despite the benefits of MIS fusion in well-selected patients,
there are limitations which include: accelerated adjacent level
degeneration, symptomatic pseudoarthrosis and graft site morbidity.
22,23 Motion sparing techniques now offer improved stability
and intersegmental motion compared to current fusion operations,
24,25 following procedures for degenerative disc disease,
spinal stenosis and spondylolisthesis.22,23,25 This allows surgeons
to avoid the aforementioned limitations, while treating patients
at earlier stages of degeneration than traditional fusion. Furthermore,
motion-sparing techniques like pedicle screw-based systems can be inserted via minimally invasive paraspinal techniques, thus
avoiding significant muscle and ligament damage.23
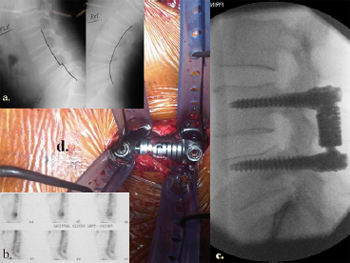
Fig. 17. Illustrative patient 8, a 54-year-old male who presented with mechanical
low back pain secondary to L4/5 facet joint arthrosis with a mobile spondylolisthesis,
resistant to multiple conservative therapies. Pedicle screw based motion
sparing techniques showing: (a) lateral image intensifier (II) radiograph showing
instability on flexion (left) and extension (right) views at L4/5. (b) Radioisotope
bone scan uptake at L4/5 facet joints. (c) Final intra-operative lateral II view of
Dynamic Stabilization Systems (DSS™, Paradigm Spine, Wurmlingen, Germany)
implant system. (d) Intra-operative photograph showing tubular dilators.
Although similar to rigid pedicle screw systems, posterior dynamic
stabilisation (PDS), including pedicle screw-based stabilisation
(Fig. 17), aims to relieve pain, other compressive neurological
symptoms, and restore stabilisation by re-establishing the natural
anatomic position and enabling restricted segmental motion.22,23
In contrast to fusion systems that aim to withstand loading until
fusion occurs, pedicle screws in dynamic stabilisation systems
need to withstand cyclical loading indefinitely, which makes the
screws prone to loosening.22
As the PDS technology has only recently emerged, the available
literature is sparse with most studies having only 2 years of followup
at most.24,25 Furthermore, it may take 5 years to 10 years before
the beneficial effects of PDS, like adjacent segment degeneration,
can be detected when compared with rigid forms of spinal fusion.
Thus, multiple, similarly designed trials need to be undertaken before
any conclusions about the benefits of PDS over current fusion
techniques can be drawn.25
5.8.1. Illustrative patient 8
A 54-year-old male presented with mechanical low back pain
secondary to L4/5 facet joint arthrosis with a mobile spondylolisthesis,
resistant to many years of multiple conservative therapies.
A posterior, motion-sparing dynamic stabilising implant was offered
as an alternative to fusion. The operating time was 2 hours,
25 minutes with 110 mL of blood loss. Follow-up at 3 months revealed
no instability on flexion/extension radiographs with moderate
reduction in low back pain scores.
6. Discussion
Since 2000, the techniques of minimally invasive spinal fusion
have improved substantially. With increasing experience, indications
for minimally invasive spinal fusion have expanded.4,10 Currently,
indications are similar to those for open surgery and
strongly rely on the surgeon's experience with the procedure.14
Most MIS spinal techniques have steep learning curves, requiring
different cognitive, psychomotor and technical skills.6,9,13 It is recommended that surgeons have adequate experience with open
procedures before attempting minimally invasive methods14 and
that they begin with simple MIS procedures. Depending on the
procedure, the patient and the surgeon's experience, MIS may take
more time to perform than open surgery.9
As indications for percutaneous pedicle screw techniques expand,
the nuances of the MIS technique will also expand. It is paramount
that experienced surgeons share their collective
knowledge to assist surgeons at their early attempts of these complex,
and potentially very dangerous procedures.
MIS aims to minimise surgery-associated risk and morbidity,
including irreversible muscle injury from muscle stripping and
retraction, which are associated with poor clinical results, while
achieving the same results as conventional approaches.6,8,10,16 Despite
encouraging clinical results, MIS techniques are in their infancy
with the results being preliminary at best. Prospective
outcome studies with long-term follow-up comparing new minimally
invasive spinal fusion to conventional open fusions are required
to ultimately determine the safety, effectiveness and
clinical benefit of minimally invasive spinal fixation.4,5,17
7. Conclusion
Spinal fusion is the gold standard in maintaining the stability of
unstable spinal segments for multiple potential pathologies. As the
techniques and instruments in MIS spinal surgery have evolved,
the indications for minimally invasive spinal fusion have expanded
to include: degeneration, trauma, deformity, infection and neoplasia.
With technological advancements, it is expected that MIS fusion
techniques will become a prominent part of spinal surgery
and that indications for minimally invasive spinal fusion will expand.
This review adds to the literature to inform prospective surgeons
of the nuances of the percutaneous technique for pedicle
screw insertion.
References
- Kanter AS, Mummaneni PV. Minimally invasive spine surgery. Neurosurg Focus
2008;25:E1.
- Mayer HM. A new microsurgical technique for minimally invasive anterior
lumbar interbody fusion. Spine 1997;22:691–700.
- Harris EB, Massey P, Lawrence J, et al. Percutaneous techniques for minimally
invasive posterior lumbar fusion. Neurosurg Focus 2008;25:E12.
- Hsieh PC, Koski TR, Sciubba DM, et al. Maximizing the potential of minimally
invasive spine surgery in complex spinal disorders. Neurosurg Focus 2008;25:
E19.
- Lipson SJ. Spinal-fusion surgery – advances and concerns. N Engl J Med
2004;350:643–4.
- Kan P, Schmidt MH. Minimally invasive thoracoscopic approach for anterior
decompression and stabilization of metastatic spine disease. Neurosurg Focus
2008;25:E8.
- Assaker R. Minimal access spinal technologies: state-of-the-art, indications, and
techniques. Joint Bone Spine 2004;71:459–69.
- Selznick LA, Shamji MF, Isaacs RE. Minimally invasive interbody fusion for
revision lumbar surgery: technical feasibility and safety. J Spinal Disord Tech
2009;22:207–13.
- Kerr SM, Tannoury C, White AP, et al. The role of minimally invasive surgery in
the lumbar spine. Oper Tech Orthop 2007;17:183–9.
- Beisse R. Endoscopic surgery on the thoracolumbar junction of the spine. Eur
Spine J 2006;15:687–704.
- Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine
2003;28:S26–35.
- Foley KT, Gupta SK. Percutaneous pedicle screw fixation of the lumbar spine:
preliminary clinical results. J Neurosurg 2002;97(Suppl. 1):7–12.
- Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine
technology and minimally invasive spine surgery: a historical review.
Neurosurg Focus 2009;27:E9.
- Holly LT, Schwender JD, Rouben DP, et al. Minimally invasive transforaminal
lumbar interbody fusion: indications, technique, and complications. Neurosurg
Focus 2006;20:E6.
- Regan JJ, Yuan H, McAfee PC. Laparoscopic fusion of the lumbar spine:
minimally invasive spine surgery. A prospective multicenter study evaluating
open and laparoscopic lumbar fusion. Spine 1999;24:402–11.
- Smith JS, Ogden AT, Fessler RG. Minimally invasive posterior thoracic fusion.
Neurosurg Focus 2008;25:E9.
- Swanson AN, Pappou IP, Cammisa FP, et al. Chronic infections of the spine:
surgical indications and treatments. Clin Orthop Relat Res 2006;444:100–6.
- Cowan JA, Thompson BG. Neurosurgery. The McGraw-Hill Companies; 2010.
Chapter 36.
- Rosen DS, Ferguson SD, Ogden AT, et al. Obesity and self-reported outcome
after minimally invasive lumbar spinal fusion surgery. Neurosurgery
2008;63:956–60.
- Vaccaro AR, Chiba K, Heller JG, et al. Bone grafting alternatives in spinal
surgery. Spine J 2002;2:206–15.
- Sandhu HS, Grewal HS, Parvataneni H. Bone grafting for spinal fusion. Orthop
Clin North Am 1999;30:685–98.
- Meyers K, Tauber M, Sudin Y, et al. Use of instrumented pedicle screws to
evaluate load sharing in posterior dynamic stabilization systems. Spine J
2008;8:926–32.
- Bertagnoli R, Fantini GA, Brau SA, et al. Spinal motion preservation technologies:
surgical approach and procedure. Chapter 22. In: Nucleus arthroplasty. Volume
IV: Emerging technologies. Minneapolis, MN, USA: Raymedica; 2007. p. 7–14.
Available from: http://www.thesona.com/V4_V5-Final.pdf.
- Heary RF. Dynamic stabilization. Neurosurg Focus 2010;28:E1.
- Bono CM, Kadaba M, Vaccaro AR. Posterior pedicle fixation-based dynamic
stabilization devices for the treatment of degenerative diseases of the lumbar
spine. J Spinal Disord Tech 2009;22:376–83.
 Technique, Challenges and Indications for Percutaneous Pedicle Screw Fixation Technique, Challenges and Indications for Percutaneous Pedicle Screw Fixation
You will need the Adobe Reader to view and print the above documents. 
|
